Key Points
CD10 identifies a unique subset of fully functional germinal center TFH that are activated and amplified within the FL cell niche.
FL CD10pos TFH specifically display an IL-4hiIFN-γlo cytokine profile and encompass the malignant B-cell-supportive TFH subset.
Abstract
In follicular lymphoma (FL), follicular helper T cells (TFH) have been depicted as one of the main components of the malignant B-cell niche and a promising therapeutic target. Although defined by their capacity to sustain FL B-cell growth together with specific gene expression and cytokine secretion profiles, FL-TFH constitute a heterogeneous cell population. However, specific markers reflecting such functional heterogeneity are still lacking. In this study, we demonstrate that CD10 identifies a subset of fully functional germinal center TFH in normal secondary lymphoid organs. Importantly, this subset is amplified in the FL context, unlike in other B-cell lymphomas with a follicular growth pattern. Furthermore, whereas FL-TFH produce high levels of interleukin (IL)-21 and low levels of IL-17 irrespectively of their CD10 expression, CD10pos FL-TFH specifically exhibit an IL-4hiIFN-γloTNF-αhi cytokine profile associated with a high capacity to sustain directly and indirectly malignant B-cell survival. Altogether, our results highlight the important role of this novel functional subset in the FL cell niche.
Introduction
The follicular lymphoma (FL) microenvironment is characterized by a strong infiltration of helper T cells displaying a complex phenotype, including an overexpression of both activation and exhaustion markers, and a specific gene expression profile (GEP), underlying altered T-cell activation, motility, and polarization.1-5 Recently, we demonstrated more precisely that genes related to follicular helper T cells (TFH), the specialized CD4pos T cells involved in normal germinal center (GC) B-cell survival and differentiation,6 represent a significant part of FL-specific microenvironment signature and revealed their unique capacity to support malignant B-cell growth.7,8 FL-TFH are regarded as a promising therapeutic target in this still incurable disease.9 FL-TFH are characterized by a specific cytokine profile, combining overexpression of interleukin (IL)-4, interferon (IFN)-γ, and tumor necrosis factor (TNF) -α, and decreased expression of helper T 17–related genes.8 However, specific markers associated with FL-TFH heterogeneity and identifying precisely the tumor-supportive FL-TFH subset are lacking.
In reactive lymphoid tissues, CD57 has been initially proposed as a marker of B-cell supportive GC-TFH,10,11 but further GEP and functional studies revealed that CD57pos and CD57neg TFH are rather similar.12 Neuropilin 1 (Nrp-1) was also detected on a subset of TFH, but no specific function could be attributed to Nrp-1pos TFH.13 Interestingly, CD10, a marker of immature T and B cells and GC B cells virtually absent on circulating mature T cells,14 has been reported on a subset of poorly characterized CD5pos T cells within reactive lymphoid hyperplasia (RLH), FL, and marginal zone lymphoma,15 as well as on malignant TFH in angioimmunoblastic T-cell lymphoma.16,17 Such results raise the possibility that CD10 expression highlights a subset of TFH within normal and malignant lymph nodes (LNs).
Combining GEP, histology, phenotype, and functional approaches, we demonstrate that CD10 expression is restricted to a unique subset of GC-TFH, specifically amplified in the FL context. Moreover, CD10pos FL-TFH exhibit a peculiar IL-4hiIFN-γlo TNF-αhi cytokine profile associated with a strong capacity to sustain directly and indirectly malignant B-cell survival.
Study design
Details are provided in the supplemental Materials and Methods (available on the Blood Web site).
Samples
Subjects were recruited under institutional review board approval and the informed consent process according to the Declaration of Helsinki. Samples comprised LNs obtained from patients with FL, nodular lymphocyte predominant Hodgkin lymphoma (NLPHL), and mantle cell lymphomas (MCLs); tonsils collected from children undergoing routine tonsillectomy; and reactive LNs with follicular hyperplasia. CD3posCD4posCXCR5hiICOShiCD25neg TFH, CD10pos TFH, and CD10neg TFH were sorted using a FACSAria (BD Biosciences) (purity >98%). Tonsil and FL B cells were purified using the human B-cell isolation kit II (Miltenyi Biotec).
Phenotypic study
Membrane and intracellular staining were performed using standard flow cytometry techniques. Data were acquired on a CyAn ADP flow cytometer and analyzed using Kaluza software (Beckman Coulter). Tissue sections were used for single immunohistochemical (Programmed cell death 1 [PD-1]), double immunohistochemical (CD10/PAX5), and double immunohistofluorescence stainings (CD10/CD3, CD10/inducible T-cell costimulator [ICOS], CD10/C-X-C motif chemokine ligand [CXCL] 13).
Microarray hybridization
GEP of 7 FL-TFH and 7 tonsil-TFH was analyzed using GeneChip HG-U133 Plus 2.0 microarrays (Affymetrix) and normalized using Partek software. Microarray data are registered to the Gene Expression Omnibus under accession number GSE66384.
FL B-cell antiapoptotic assay
Purified FL malignant B cells were cultured alone, with an activation cocktail (CD40 ligand, IL-2, IL-4), or in the presence of purified CD10pos or CD10neg TFH (ratio 1:1). After 48 hours, B-cell apoptosis was assessed on CD20posCD2neg B cells using active caspase-3 phycoerythrin apoptosis kit (BD Biosciences).
Statistical analyses
Statistical analyses were performed with the GraphPad Prism software using nonparametric Wilcoxon test for matched pairs, or Mann-Whitney U tests.
Results and discussion
Scattered PAX5negCD10hi cells could be identified within neoplastic follicles in 16/19 FL samples and were characterized as CD3posCD4pos T cells with a mature GC-TFH phenotype, that is, expressing high levels of C-X-C motif chemokine receptor (CXCR) 5 and PD-1, together with ICOS and CXCL13 (Figure 1A-C). FL-infiltrating PD-1negCXCR5neg non-TFH and PD-1intCXCR5int pre-TFH, as well as blood CD4posCD45RAnegCXCR5pos T cells representing circulating memory FL-TFH did not express CD10 (Figure 1B and data not shown). To evaluate if CD10 expression on TFH was FL specific, we tested other B-cell lymphomas with a follicular growth pattern. Whereas cells expressing PD-1 were rarely detected in MCL samples, in agreement with a lack of PAX5negCD10pos T cells, we confirmed the presence of numerous PD-1pos cells forming rosettes around neoplastic cells in NLPHL, but these cells were essentially CD10neg (data not shown). The lack of MCL-infiltrating TFH and the absence of CD10 expression on PD-1pos T cells in the NLPHL microenvironment were confirmed by flow cytometry (data not shown). By contrast, we identified PAX5negCD10pos T cells at a highly variable frequency within the GC of all RLHs. These cells tended to concentrate at the periphery of the GC and coexpressed CD3, ICOS, and CXCL13 (supplemental Figure 1A-B). To evaluate how CD10 expression could impact TFH function, we first sorted paired CD10pos and CD10neg TFH from tonsils and demonstrated similar expression of canonical TFH genes (supplemental Figure 1C). Moreover, CD10pos and CD10neg TFH displayed the same capacity to sustain immunoglobulin production by autologous purified B cells (supplemental Figure 1D). We next checked whether CD10pos tonsil-TFH could be enriched for CD57- or Nrp-1-expressing cells (supplemental Figure 1E). As reported for Nrp-1,13 CD10 was expressed by a higher proportion of CD57pos than CD57neg TFH (22.2% [6.1% to 51.4%] vs 15.6% [3.4% to 35.2%], P < .001, n = 20). However, the expression of CD10 and Nrp-1 was mutually exclusive. Finally, CD10 was not expressed on CXCR5hiICOShiFoxp3posCD25pos tonsil follicular regulatory T cells. Thus, CD10 identifies a distinct subset of fully functional GC-TFH in human RLH. Interestingly, conversely to NRP1 and CD57, CD10/MME was significantly upregulated in FL-TFH compared with tonsil-TFH, as revealed by GEP analysis. Accordingly, the percentage of CD10pos TFH was increased in FL samples (6.17% [2.7% to 22.9%]) compared with tonsils (1.56% [0.2% to 10.1%], P < .001) (Figure 1D-E), raising the hypothesis that CD10pos TFH expansion could play a specific role in FL pathogenesis.
Characterization of CD10pos T cells in B-cell lymphomas. (A) Double staining revealed the presence of scattered strong CD10pos cells within FL neoplastic follicles. Numerous CD10pos (brown) PAX5neg (red) lymphocytes are evidenced (arrows). Original magnification: objective ×20 (left) and ×40 (right). (B) Representative characterization of ICOS, CD10, CXCR5, and PD-1 staining by flow cytometry on CD3posCD4pos viable cells from FL LNs. (C) In situ characterization of CD10pos T cells in RLH including reactive LNs and tonsils, and B-cell lymphomas with follicular growth pattern (MCL, NLPHL, and FL). For each sample, the numbers of CD10posPAX5neg and PD-1pos cells found inside GCs were counted in 10 high power fields (×40), and the median of these 10 values was calculated. Data are expressed as the median [range] of the different samples tested. ND, not done. (D) Expression of MME/CD10 in CD3posCD4pos CXCR5highICOShighCD25neg TFH purified from 7 reactive tonsils and 7 FL samples (203435_s_at probeset of the GeneChip HG-U133 Plus 2.0 oligonucleotide arrays). **P < .01. (E) Frequency of CD10pos TFH among CD4pos T cells in 15 FL LNs and 25 reactive tonsil samples, evaluated by flow cytometry. ***P < .001.
Characterization of CD10pos T cells in B-cell lymphomas. (A) Double staining revealed the presence of scattered strong CD10pos cells within FL neoplastic follicles. Numerous CD10pos (brown) PAX5neg (red) lymphocytes are evidenced (arrows). Original magnification: objective ×20 (left) and ×40 (right). (B) Representative characterization of ICOS, CD10, CXCR5, and PD-1 staining by flow cytometry on CD3posCD4pos viable cells from FL LNs. (C) In situ characterization of CD10pos T cells in RLH including reactive LNs and tonsils, and B-cell lymphomas with follicular growth pattern (MCL, NLPHL, and FL). For each sample, the numbers of CD10posPAX5neg and PD-1pos cells found inside GCs were counted in 10 high power fields (×40), and the median of these 10 values was calculated. Data are expressed as the median [range] of the different samples tested. ND, not done. (D) Expression of MME/CD10 in CD3posCD4pos CXCR5highICOShighCD25neg TFH purified from 7 reactive tonsils and 7 FL samples (203435_s_at probeset of the GeneChip HG-U133 Plus 2.0 oligonucleotide arrays). **P < .01. (E) Frequency of CD10pos TFH among CD4pos T cells in 15 FL LNs and 25 reactive tonsil samples, evaluated by flow cytometry. ***P < .001.
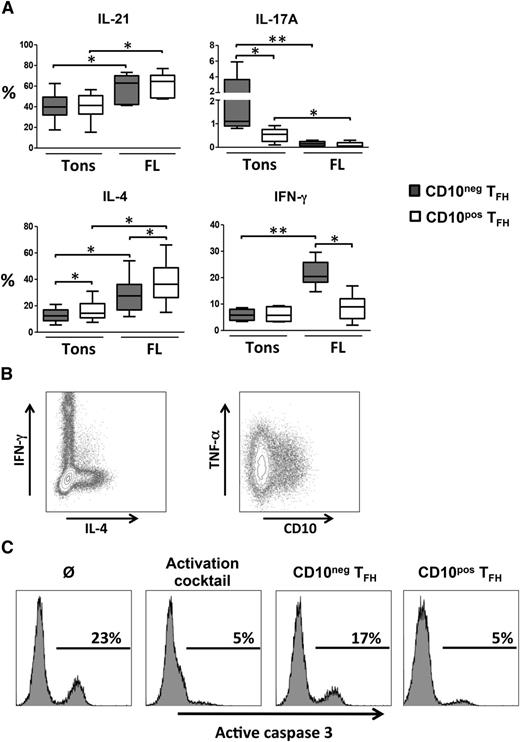
CD10pos FL-TFH were essentially Ki-67neg but expressed high levels of HLA-DR indicating a nonproliferating but activated status (supplemental Figure 2). In addition, neither CD10pos nor CD10neg FL-TFH expressed T cell immunoglobulin mucin-3 (TIM-3) confirming that they represent fully activated and not exhausted T cells (data not shown). We next looked for the capacity of TFH to produce cytokines after in vitro restimulation. Interestingly, CD10pos tonsil-TFH produced similar levels of IL-21 and IFN-γ but significantly less IL-17A and more IL-4 than their CD10neg counterpart, indicating that CD10 highlights a unique subset of TFH with specific functional properties (Figure 2A). Compared with tonsil-TFH, FL-TFH displayed an IL-21hiIL-17lo phenotype independently of their CD10 expression. However, CD10pos FL-TFH exhibited a higher capacity to secrete IL-4 and a reduced capacity to secrete IFN-γ compared with CD10neg FL-TFH. In agreement, IL-4-producing FL-TFH and IFN-γ-producing FL-TFH belonged to nonoverlapping cell subsets (Figure 2B). These results prompted us to evaluate the direct role of CD10pos vs CD10neg FL-TFH on malignant FL B cells. CD10pos FL-TFH were more efficient than their CD10neg counterpart to support autologous malignant FL B-cell survival in vitro (Figure 2C). Finally, the previously reported overexpression of TNFA by FL-TFH8 appeared uniformly supported by CD10pos and CD10neg FL-TFH subsets, containing both ∼35% of TNF-α-producing cells. Altogether, our data suggest that these 2 FL-TFH subsets may have different roles within the FL cell niche. CD10pos FL-TFH produce high amounts of IL-4 that could trigger B-cell activation, survival, and production of the Treg-recruiting CC chemokine ligands 17 and 22.8,18 IL-4 also contributes to the polarization of tumor-associated macrophages19 that favor FL B-cell growth.20,21 In addition, CD10pos FL-TFH exhibit a TNFhiIFNγlo phenotype that could favor the induction and maintenance of a B-cell supportive lymphoid stroma network.22 Conversely, overexpression of IFN-γ by CD10neg TFH could promote activation of cytotoxic CD8pos T cells displaying efficient antitumor activity23 but also expression of stroma-derived indoleamine-2,3 dioxygenase that could inhibit not only T-cell but also malignant B-cell proliferation.24
Functional characterization of CD10pos and CD10neg FL-TFH. (A) FL LN or reactive tonsil (Tons) cells were stimulated with phorbol 12-myristate 13-acetate and ionomycin for 6 hours, and with brefeldin A for the last 4 hours of stimulation before intracytoplasmic detection of cytokines. The percentage of singlet viable CD10neg (gray) or CD10pos (white) viable TFH producing IL-21, IL-17A, IL-4, and IFN-γ was determined. *P < .05, **P < .01. (B) Representative plots of IFN-γ/IL-4 and CD10/TNF-α staining on FL-TFH. (C) Purified malignant FL B cells were cultured alone (Ø), with an activation cocktail (recombinant human CD40 ligand, IL-2, and IL-4), or in the presence of autologous CD10neg or CD10pos TFH at a 1:1 ratio for 48 hours. Representative plots of active caspase-3 staining gated on CD20pos B cells (n = 3).
Functional characterization of CD10pos and CD10neg FL-TFH. (A) FL LN or reactive tonsil (Tons) cells were stimulated with phorbol 12-myristate 13-acetate and ionomycin for 6 hours, and with brefeldin A for the last 4 hours of stimulation before intracytoplasmic detection of cytokines. The percentage of singlet viable CD10neg (gray) or CD10pos (white) viable TFH producing IL-21, IL-17A, IL-4, and IFN-γ was determined. *P < .05, **P < .01. (B) Representative plots of IFN-γ/IL-4 and CD10/TNF-α staining on FL-TFH. (C) Purified malignant FL B cells were cultured alone (Ø), with an activation cocktail (recombinant human CD40 ligand, IL-2, and IL-4), or in the presence of autologous CD10neg or CD10pos TFH at a 1:1 ratio for 48 hours. Representative plots of active caspase-3 staining gated on CD20pos B cells (n = 3).
In conclusion, our study supports the current understanding of the FL cell niche as an intricate network of cell interactions in which each cell subset should be exquisitely characterized individually and in relationship with the other partners before being proposed as a biomarker or therapeutic target.
The data reported in this article have been deposited in the Gene Expression Omnibus database (accession number GSE66384).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Labex Immunotherapy Graft Oncology, the Centre de Ressources Biologiques–Santé (BB-0033-00056; http://www.crbsante-rennes.com) of Rennes Hospital for its support in the processing of biological samples, the Unité Mixte de Service Centre National de la Recherche Scientifique (CNRS) 3480/US INSERM 018 Biologie Santé Innovation Technologique (BIOSIT) for cell-sorting core facility, Christophe Ruaux for providing tonsil samples, and Christiane Copie and Maryse Baia for slide-scanning facility.
This work was supported by research funding from the Institut National du Cancer (INCA AAP PLBIO-13-085 and INCA_6530), the Ligue Nationale Contre le Cancer (Equipe Labellisée and “Carte d'identité des Tumeurs” program), and the Fondation “Association pour la Recherche contre le Cancer.” J.M. has been funded by FP7-PEOPLE-2011-Initial Training Network Stroma Cell-Immune Cell Interactions in Health and Disease (STROMA).
Authorship
Contribution: P.A.-T. designed and performed research, analyzed data, and wrote the manuscript; S.H. designed and performed research, analyzed data, and corrected the manuscript; C.A., J.M., M.S.B., R.J., J.L.P., C.M., and N.M. performed research; and P.G. and K.T. supervised and designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: S.H. was supported by the Novartis Foundation (Basel, Switzerland). The remaining authors declare no competing financial interests.
Correspondence: Philippe Gaulard, INSERM U955, Centre Hospitalier Universitaire Henri Mondor, Faculté de Médecine de Créteil 8 rue du Général Sarrail, F-94010 Créteil, France; e-mail: philippe.gaulard@hmn.aphp.fr; and Karin Tarte, INSERM U917, Faculté de Médecine, 2 Ave du Pr Léon Bernard, F-35043 Rennes, France; e-mail: karin.tarte@univ-rennes1.fr.
References
Author notes
P.A.-T. and S.H. contributed equally to this study.
P.G. and K.T. contributed equally to this study.

![Figure 1. Characterization of CD10pos T cells in B-cell lymphomas. (A) Double staining revealed the presence of scattered strong CD10pos cells within FL neoplastic follicles. Numerous CD10pos (brown) PAX5neg (red) lymphocytes are evidenced (arrows). Original magnification: objective ×20 (left) and ×40 (right). (B) Representative characterization of ICOS, CD10, CXCR5, and PD-1 staining by flow cytometry on CD3posCD4pos viable cells from FL LNs. (C) In situ characterization of CD10pos T cells in RLH including reactive LNs and tonsils, and B-cell lymphomas with follicular growth pattern (MCL, NLPHL, and FL). For each sample, the numbers of CD10posPAX5neg and PD-1pos cells found inside GCs were counted in 10 high power fields (×40), and the median of these 10 values was calculated. Data are expressed as the median [range] of the different samples tested. ND, not done. (D) Expression of MME/CD10 in CD3posCD4pos CXCR5highICOShighCD25neg TFH purified from 7 reactive tonsils and 7 FL samples (203435_s_at probeset of the GeneChip HG-U133 Plus 2.0 oligonucleotide arrays). **P < .01. (E) Frequency of CD10pos TFH among CD4pos T cells in 15 FL LNs and 25 reactive tonsil samples, evaluated by flow cytometry. ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/15/10.1182_blood-2015-02-625152/4/m_2381f1.jpeg?Expires=1767755138&Signature=0HYnx95iZqoOeLFVdjuPvvRerI33zzitzGGFiOgMA2XcCD1u5kxUPhisoXjrT67gh1TPcT0PNbily-CZj9KRafX1gSe7L1LPPeqCSdyIYloK4DEEqWkNHArAR49v-z5aP--Nye9qDCtlzuds3PvTu4XjjbU3DfmwHHL11m8Kjjwp0Krq7R08HjA-c5Bmjf4iSqd1IEoTAcXZfmlvFEGNIM5ehNTdR27qgJYtOrnizBM2VByyUp7WiXGmwzEZgXCY54b5gIFjMKqAw-tVaYRA27gU5MqnGctgSLGr11jTfcmoYnyHQyvxv64bNMtnNTGkYhYruZektTy2B4apbB20uA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 1. Characterization of CD10pos T cells in B-cell lymphomas. (A) Double staining revealed the presence of scattered strong CD10pos cells within FL neoplastic follicles. Numerous CD10pos (brown) PAX5neg (red) lymphocytes are evidenced (arrows). Original magnification: objective ×20 (left) and ×40 (right). (B) Representative characterization of ICOS, CD10, CXCR5, and PD-1 staining by flow cytometry on CD3posCD4pos viable cells from FL LNs. (C) In situ characterization of CD10pos T cells in RLH including reactive LNs and tonsils, and B-cell lymphomas with follicular growth pattern (MCL, NLPHL, and FL). For each sample, the numbers of CD10posPAX5neg and PD-1pos cells found inside GCs were counted in 10 high power fields (×40), and the median of these 10 values was calculated. Data are expressed as the median [range] of the different samples tested. ND, not done. (D) Expression of MME/CD10 in CD3posCD4pos CXCR5highICOShighCD25neg TFH purified from 7 reactive tonsils and 7 FL samples (203435_s_at probeset of the GeneChip HG-U133 Plus 2.0 oligonucleotide arrays). **P < .01. (E) Frequency of CD10pos TFH among CD4pos T cells in 15 FL LNs and 25 reactive tonsil samples, evaluated by flow cytometry. ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/15/10.1182_blood-2015-02-625152/4/m_2381f1.jpeg?Expires=1767755139&Signature=C-pDEp-oHPI-DB6~~Fr4m6yjpN3dNeI71ycGctGV2ax4W5Y0yuqA97i8xum4LnwXoMwM9qUAV0U68Qwi1X5mg3whnkQbtRtZsI-8x-ywkIYp3NsYtWUmqDdJC5BfFuZvdscuRti0Ukr3CNLzDkpmjbPaRBVaK330wZVK6LfvHNbiZU-e0kAIyGWfRJZAYKuYnYworGv4L2qGs5s74yaMFY9IaDWXmW32iq0ibIm0b8NzzqBMmTXqtpOXq1VcQWDYV-a3pxU-3CuJHJy3lCoaPcfcAM1T2TCFY02YXsFcw7pnFp6zz2uU0wl9giydtikJruz-hEdr5e0jXNh11DxCkQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)