Abstract
Mixed-phenotype acute leukemia (MPAL) encompasses a heterogeneous group of rare leukemias in which assigning a single lineage of origin is not possible. A variety of different terms and classification systems have been used historically to describe this entity. MPAL is currently defined by a limited set of lineage-specific markers proposed in the 2008 World Health Organization monograph on classification of tumors of hematopoietic and lymphoid tissues. In adult patients, MPAL is characterized by relative therapeutic resistance that may be attributed in part to the high proportion of patients with adverse cytogenetic abnormalities. No prospective, controlled trials exist to guide therapy. The limited available data suggest that an “acute lymphoblastic leukemia–like” regimen followed by allogeneic stem-cell transplant may be advisable; addition of a tyrosine kinase inhibitor in patients with t(9;22) translocation is recommended. The role of immunophenotypic and genetic markers in guiding chemotherapy choice and postremission strategy, as well as the utility of targeted therapies in non–Ph-positive MPALs is unknown.
Introduction
The absence of essentially any useful prospectively collected data on how to treat mixed-phenotypic acute leukemia (MPAL) in adults both simplifies and complicates any discussion of this topic. Given the availability of little truly useful information, we have derived an approach based on data in the literature that makes logical sense and can be adhered to: once MPAL is definitely identified, patients should be treated according to an “acute lymphoid leukemia”–type induction regimen followed by allogeneic stem-cell transplant (alloSCT) in responding patients if feasible.
Case presentation: part 1
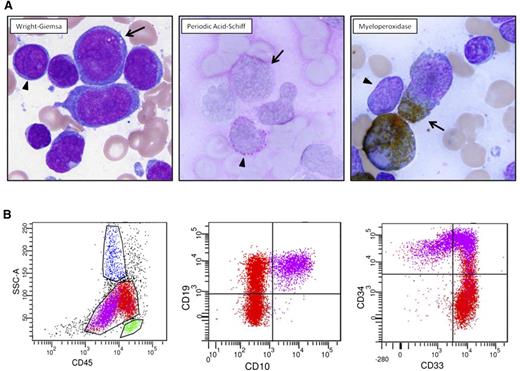
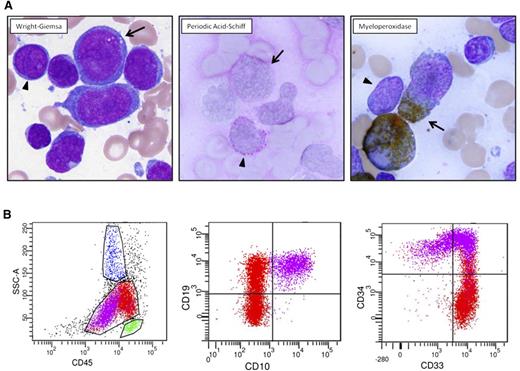
A 51-year-old physician complained of 2 weeks of dyspnea upon exertion. White blood cell (WBC) count was 280 × 109/L (76% blasts), platelet count was 91 × 109/L, and hemoglobin was 9 g/dL. Bone-marrow aspirate (Figure 1A) revealed 2 distinct morphologic/cytochemical populations of blasts that were positive for myeloperoxidase (MPO) and periodic acid-Schiff (PAS) stains, respectively. Flow cytometry (Figure 1B) also demonstrated 2 atypical blast populations with distinct CD45 expression associated with (1) uniform expression of B-lymphoid markers CD19 and CD10, uniform stem-cell marker CD34, and variable myeloid marker CD33; and (2) uniform expression of CD33, small subset CD34, variable CD19, and lack of CD10 expression.
Diagnostic pathology of clinical case. (A) Two atypical blast populations are seen on bone marrow aspirate smear. One population (arrowhead) is composed of small cells with round nuclei, slightly condensed chromatin, distinct nucleoli, and scant cytoplasm that shows cytoplasmic reactivity with periodic acid-Schiff (PAS) in a blocklike pattern and lacks reactivity with myeloperoxidase (MPO). The other population (arrow) is composed of large cells with irregular nuclei, dispersed chromatin, variably distinct nucleoli, and small-to-moderate amounts of blue-gray cytoplasm that shows cytoplasmic reactivity with MPO and lacks reactivity with PAS. (B) Flow cytometric analysis of this bone marrow aspirate reveals 2 atypical blast populations (one highlighted in purple, one highlighted in red) with distinct CD45 expression and variable antigen expression profiles. The CD45(dim) purple population exhibits uniform expression of B-lymphoid markers CD19 and CD10, uniform stem-cell marker CD34, and variable myeloid marker CD33. The red population shows brighter CD45 expression, exhibits uniform expression of CD33, small subset CD34, variable CD19, and lacking CD10 expression.
Diagnostic pathology of clinical case. (A) Two atypical blast populations are seen on bone marrow aspirate smear. One population (arrowhead) is composed of small cells with round nuclei, slightly condensed chromatin, distinct nucleoli, and scant cytoplasm that shows cytoplasmic reactivity with periodic acid-Schiff (PAS) in a blocklike pattern and lacks reactivity with myeloperoxidase (MPO). The other population (arrow) is composed of large cells with irregular nuclei, dispersed chromatin, variably distinct nucleoli, and small-to-moderate amounts of blue-gray cytoplasm that shows cytoplasmic reactivity with MPO and lacks reactivity with PAS. (B) Flow cytometric analysis of this bone marrow aspirate reveals 2 atypical blast populations (one highlighted in purple, one highlighted in red) with distinct CD45 expression and variable antigen expression profiles. The CD45(dim) purple population exhibits uniform expression of B-lymphoid markers CD19 and CD10, uniform stem-cell marker CD34, and variable myeloid marker CD33. The red population shows brighter CD45 expression, exhibits uniform expression of CD33, small subset CD34, variable CD19, and lacking CD10 expression.
All 20 metaphases assessed contained a t(9;22)(q34;q11.2) translocation associated with loss of the 7p and 16q arms. Molecular studies demonstrated the e1a2 BCR-ABL transcript (p190) but no additional recurrent mutations associated with acute leukemia (95-gene panel).
What is mixed-phenotype acute leukemia?
Patients diagnosed with acute leukemia (>20% blasts in blood or marrow, or fewer in the case of certain chromosomal translocations or an extramedullary presentation) can generally be classified as having either myeloid lineage–derived disease (AML) or lymphoid lineage–derived disease (ALL). Sometimes the immature cells display cytochemical and/or immunophenotypic features of both lineages (biphenotypic) or there are different populations of leukemia cells (bilineal). The distinction between bilineal and biphenotypic leukemias is often blurred, especially because 2 “populations” of cells perhaps represent subclones derived from a unique stem cell. Accordingly, this distinction does not generally affect our diagnostic or therapeutic approach.
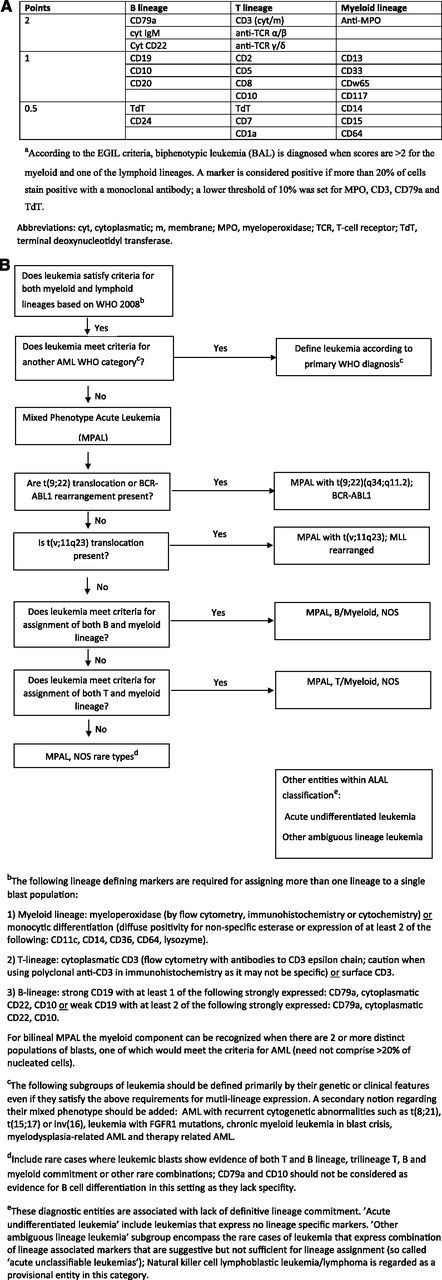
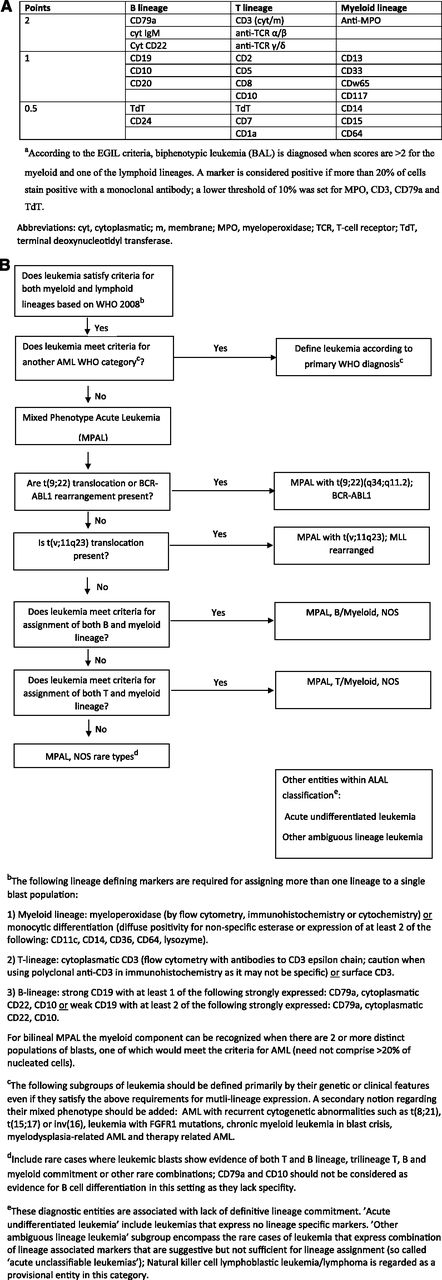
Two important recent algorithms have been used to define this entity. In the first of these (1995), the European Group for Immunological Characterization of Acute Leukemias (EGIL) developed a scoring algorithm in which a point system determined whether a patient had enough immunophenotypic variety to qualify as biphenotypic (Figure 2A).1,2 The second and most recent 2008 World Health Organization (WHO) monograph on classification of tumors of hematopoietic and lymphoid tissues includes a helpful chapter on acute leukemias of ambiguous lineage: “leukemias that show no clear evidence of differentiation along a single lineage.”3 These encompass MPAL, the primary topic of this review, and acute undifferentiated leukemia (AUL), wherein the malignant cells do not express lineage-specific antigens. This classification (Figure 2B) tries to minimize the difficult distinction between bilineal and biphenotypic leukemia (BAL) and subclassifies these promiscuously-derived cells as usually either B-myeloid or T-myeloid. MPALs that harbor Philadelphia chromosome (Ph+) or MLL rearrangements are considered a distinct diagnostic subgroup (Figure 2B). An important point is that AML-defining balanced translocations such as t(8;21), a type of favorable prognosis AML that frequently expresses multiple B-cell markers,4 are not considered biphenotypic. It also excludes secondary leukemias (arising after prior cancer therapy or myelodysplasia), leukemias with FGFR1 mutations that have features of both T-lymphoid and myeloid differentiation, and chronic myeloid leukemia (CML) in blast crisis, which can present with a variety of lineages. The latter is sometimes difficult to separate from Ph+ MPAL (that may actually represent transformation from a previously undiagnosed chronic-phase CML).
Diagnostic criteria for BAL and MPAL. (A) EGIL criteria for the diagnosis of biphenotypic acute leukemia.a (B) 2008 WHO criteria. Leukemias that fail to demonstrate differentiation along a single lineage are defined as acute leukemias of ambiguous lineage (ALAL) and are further subdivided into diagnostic subgroups. A practical approach for the diagnosis of MPAL is presented.
Diagnostic criteria for BAL and MPAL. (A) EGIL criteria for the diagnosis of biphenotypic acute leukemia.a (B) 2008 WHO criteria. Leukemias that fail to demonstrate differentiation along a single lineage are defined as acute leukemias of ambiguous lineage (ALAL) and are further subdivided into diagnostic subgroups. A practical approach for the diagnosis of MPAL is presented.
The essential feature of MPAL (Figure 2B) is that cells express lineage-specific myeloid markers as well as lineage-specific T- or B-lymphoid markers. Although there are caveats (see legend to Figure 2B), CD3 expression equals T-lymphoid development, and CD19 plus 1 or 2 other markers suggests B-lymphoid origin. Myeloid origin can be determined with a set of monocytic markers, or more commonly by MPO expression. Although various thresholds for flow-based MPO positivity were introduced over the years (eg, 10% of blast population1,5 ), no specific threshold has been acknowledged in the 2008 WHO monograph.3,6
Compared with the EGIL classification, the 2008 WHO classification uses a more limited set of lineage markers that can be more consistently applied. In 2015, the 2008 WHO classification still remains the most practical means to define and subclassify MPAL, but it is hoped that advances in deciphering the molecular pathogenesis of acute leukemia will soon lead to a more robust approach to the diagnosis of these entities.
What drives biphenotypic expression in leukemia?
During the 1980s, 2 leading hypotheses were raised to explain biphenotypic expression in leukemia. The Greaves hypothesis suggested “lineage promiscuity,” in which hematopoietic progenitor cells possess multilineage potential that is preserved as a relic if leukemic transformation occurs at that stage.7 The term “lineage infidelity” denoted an alternative hypothesis involving oncogenetically-driven misprogramming of the leukemic cell, resulting in multilineage-expressing blasts.8,9 Significant strides have been made since then in our understanding of the normal and pathologic pathways that drive lineage fate.
Maturation and differentiation of blood cells during the process of hematopoiesis is associated with the expression of specific sets of markers that define lineage. This is a tightly regulated, multistep, hierarchical process driven by a network of transcription factors. Although some transcription factors are thought to have primary roles in driving hematopoietic progenitors toward a specific lineage (eg, C/EBPα in myeloid cells or PAX-5 in B lymphocytes), this relationship in vivo is far more complex, context dependent, and regulated at multiple cellular levels.10-12 Early hematopoietic multipotential progenitors were previously shown to express markers of multiple lines, with the specific fate selection relying on complex interactions that both promote a specific lineage phenotype and also suppress alternative programming (so called “lineage priming”).13 The timing and level of expression of a specific transcription factor may affect lineage determination. Competing transcription factors interact to antagonize each other’s functions to promote the expression of one lineage over the other.11 For example, a high level of PAX5 expression is critical for development of common lymphoid progenitors along the B-cell pathway, whereas low levels result in a mixed phenoptype14 ; C/EBPα suppression of PAX-5 drives common lymphoid progenitor cells toward myeloid phenotypes.15 In zebrafish PU.1 and GATA-1 exert mutually antagonistic effects with the balance driving myeloid vs erythroid differentiation.16 The fate of early T-cell lineage progenitors is dependent on the Notch receptor–signaling pathway, without which myeloid differentiation may occur.17-19
Dysregulation and aberrant expression of transcription factors that govern cell differentiation occur on the basis of the genomic and epigenetic alterations seen in acute leukemia.11,20 Gene-expression profiling in a large group of patients with AML correlated T/myeloid phenotype with a distinct expression profile that included C/EBPα promoter hypermethylation/gene silencing and upregulation of T cell–lineage pathways, via aberrantly activated NOTCH1 signaling.21 Activating mutations in NOTCH1 were previously described in the context of lineage switch from AML to T-ALL, suggesting the potential role of mutations in transcription factors on lineage-specific cell reprogramming.22 Indeed, mutations may trump phenotype. Early T-cell precursor (ETP) ALL is associated with recurrent mutations typically seen in myeloid tumors such as DNMT3A, IDH1, and IDH223-25 and is transcriptionally related to myeloid progenitors.
Natural history of MPAL
Incidence
The frequency, clinical features, and outcome of patients with ambiguous lineage expression are largely dependent on the classification system used at the time of report. The WHO 2008 classification is less inclusive than the preceding EGIL system, resulting in a lower reported prevalence. Weinberg and Arber retrospectively reviewed series encompassing 7627 pediatric and adult patients with acute leukemia and determined that 2.8% had BAL and 1.6% had MPAL using the EGIL and WHO 2008 systems, respectively.26 A more recent Chinese study reported MPAL in 2.4% of 4780 patients with acute leukemia (ages 14-81 years).27 In 517 pediatric and adult Dutch patients with acute leukemia, 30 patients (5.8%) would be considered as having BAL based on EGIL criteria, and 8 cases (1.5%) were consistent with MPAL using the WHO 2008 classification; only 6 patients (1.1%) would qualify as both BAL and MPAL, suggesting that these classification systems may select different patients.28
Characteristics of MPAL
Matutes et al29 presented a review of 100 patients, mostly from the United Kingdom and Austria, with MPAL based on the WHO definition. Of the 62 men and 38 women (32% under the age of 16), 39 displayed ALL, 38 had AML, and 13 cases were defined as acute undifferentiated leukemia by morphologic assessment (10 were not analyzed). Immunophenotyping showed that 58% of the cases had a B-myeloid and 36% had a T-myeloid phenotype. Combined B+T and trilineage (myeloid+B+T) immunophenotypes were rare (n = 6) and all of these had ALL morphology. Expression of stem cell–like markers was common and included terminal deoxynucleotidyl transferase in 89% of the cases, human leukocyte antigen–D-related in 92% and CD34 in 74%. Among cases with myeloid commitment, MPO was expressed in at least in 5% of the blasts in 98% of cases, and in >20% of the blasts in 76% of the cases. All except 9 cases expressed MPO, as well as CD33 and/or CD13. By definition, cytoplasmic CD3 was expressed in all 35 cases with T-myeloid phenotype and CD19 was present in 93% of cases with the B-myeloid phenotype and was always associated with CD10, cytCD22, and/or cytCD79a expression. In the 76 patients with cytogenetic information, 20% had a Ph+ and 8% had MLL gene (11q23) rearrangements. Thirty-two percent had complex karyotype (CK) that was commonly associated with deletion of the long arm of chromosome 6, abnormalities involving the long arm of chromosome 7, or abnormalities in the long arm of chromosome 5. Normal karyotype was demonstrated in 13%. Although early deaths were seen, most of the patients died of their disease, with an overall median survival of 18 months and a 37% overall 5-year survival. Age, Ph+, and the type of induction therapy were significant predictors for survival, with children surviving 139 months vs 11 months for adults, 8 months for Ph+ vs 139 months for those with normal karyotype, and 28 months for those with other abnormalities. Yan et al27 reported on 117 patients with WHO 2008–defined MPAL. Median patient age was 35 years (range 14-81) with a slight male predominance (51.3%) and a median WBC count of 5.4 × 109/L (range 0.8-278.7) at diagnosis. Thirty-four percent of patients demonstrated AML morphology (primarily FAB M1 and FAB M5), 44% were believed to have ALL (FAB L1), and 22% were unclassifiable. B-myeloid immunophenotype was seen in 55% and T-myeloid phenotype in 33%. Of 92 patients assessed, 64% presented with cytogenetic abnormalities; CK was the most prevalent aberration found in 24% of patients, followed by Ph+ chromosome in 15% (all B-myeloid phenotype) and translocations involving MLL gene at 11q23 in 4.3% of patients. Monosomy 7, polysomy 21, and trisomy 8 were also noted in a significant minority of patients.
Cytogenetic abnormalities in MPAL and BAL were reported in a recent systematic review to be present in 59% to 91% of patients.30 The prevalence of Ph+ and complex karyotype increases with age. The frequency of translocations involving 11q23 (usually MLL-AF4 or MLL-ENL fusions) decreases with age and is quite uncommon in adults with MPAL.30 One could argue that leukemia with CK or other myelodysplastic-specific cytogenetic abnormalities should be classified as AML with myelodysplasia-related changes rather than MPAL.
Genetic alterations in MPAL
Rubnitz et al31 analyzed gene expression patterns in 13 pediatric patients with EGIL-defined BAL and found that, although 5 patients made up a group with known AML expression patterns, 8 patients displayed gene expression patterns that were different from AML and ALL, suggesting that some cases of BAL may be a biologically distinct entity. In contrast, microRNA profiling studies suggest that MPAL does not appear to be a distinct entity. de Leeuw et al32 analyzed 16 cases of acute leukemia of ambiguous lineage and demonstrated that all cases had microRNA expression profiles that clustered with AML or ALL. Heesch et al 33 noted a higher expression of BAALC and ERG—adverse prognostic characteristics in AML—in 26 cases of EGIL-defined BAL compared with other cases of AML.
Information regarding the mutational landscape of MPAL is based on small patient numbers. Yan et al27 analyzed 31 patients with MPAL for 18 leukemia-related mutations and reported that 12 patients (39%) were found to harbor a mutation, including IKZF1 deletion in 4 patients (all B-myeloid phenotype), EZH2 in 3 (B- or T-myeloid), ASXL1 in 2 (both B-myeloid), TET2 in one (B-myeloid), and ETV6 and NOTCH1 in 1 patient each (both T-myeloid). A high rate of DNMT3A mutations was reported in adults with T-myeloid MPAL (10/18 patients; mostly biallelic mutations).34 Whole-exome sequencing in 19 adult patients with MPAL (12 T-myeloid, 6 B-myeloid, and 1 B/T) demonstrated that 63% of patients had mutations in epigenetic regulatory genes. DNMT3A was the most common mutation (n = 6) followed by EZH2, IDH1/2, TET1, and TET3. Other recurrent mutations included PRPF40B (n = 6), TP53 (n = 5), BRAF (n = 4), and NOTCH1 (n = 4).35 Some of these mutations (eg, IDH1/2, BRAF, NOTCH1, FLT3) could be theoretically targeted by available agents or those in current clinical trials. In another series, clustering of FLT3 ITD and TKD mutations was reported in patients with T-myeloid MPAL. Seven of 15 patients (47%) were positive for FLT3 mutations (mostly ITD), all of which were CD117+.36 Array-based comparative genomic hybridization analysis in 12 patients with MPAL demonstrated that all patients had at least 1 abnormality, including deletions of CDKN2A, IKZF1, MEF2C, BCOR, EBF1, KRAS, LEF1, MBNL1, PBX3, and RUNX1.27
Risk factors and outcomes
The reasons underlying resistance to therapy in this heterogeneous group are not clear but may be related to the high prevalence of drug efflux pump expression37-39 and the high proportion with cytogenetic abnormalities.30 Whatever classification is used, there appears to be a uniformly poor outcome in MPAL (or BAL) that is inferior to the outcome in more typical AML or ALL. Based on adult and mixed pediatric/adult series, patients with EGIL-defined BAL have complete remission (CR) rates of 30% to 80.6%,33,37,40-46 with median disease-free survival (DFS) and overall survival (OS) of 5 to 12 months37,44-46 and 6.5 to 30.3 months,33,37,40,41,44-46 respectively. In the few larger retrospective series of WHO 2008–defined MPAL, CR rates are reported at 61.5% to 85.2%,27,29,47,48 and median OS is reported to be 14.8 to 18 months.29,47 Factors associated with outcome in these analyses include age,29,31,37,40,44,48-50 WBC count at diagnosis,41,44 Ph+ status,29,40 CK or MLL rearrangement,45 baseline creatinine and uric acid levels,47 extra medullary involvement at diagnosis,44 immunophenotype (T-myeloid being worse),41 failing to respond to induction therapy,37,44,48 type of induction therapy (favoring non-AML),29,44,48 and type of postremission therapy (favoring transplant27,33 and more intensive conditioning48 ).
Treatment of MPAL
There are no prospective trials that point to an optimal strategy. Beyond one’s own experience, we are left with heterogeneous case series that describe outcomes retrospectively. Further complicating data interpretation is the inclusion of patients with well-defined AML syndromes in previous classifications (such as core-binding-factor leukemias) that may bias those reports toward the use of AML-type therapy. Case studies from individual centers or countries tend to examine all cases of acute leukemia and describe MPAL in 2% to 3% of the population. Although these studies probably reflect the true incidence of the entity fairly accurately, treatment decisions are haphazard and are subject to unknown bias regarding individual physicians, because there was no widespread treatment policy. The few studies that retrospectively garnered MPAL cases from cooperative group trials used more homogeneous treatments, but inevitably excluded cases from eligibility based on ambiguity. Neither is there much guidance in the National Comprehensive Cancer Network (NCCN) guidelines. The precise definition of which cases should be treated according to the ALL vs AML guidelines is sidestepped. The reader is advised to consult a center or individual with experience in diagnosing these entities.51 As someone who serves on the AML NCCN guidelines committee, I (R.M.S.) readily admit personal uncertainty, which can be blamed on the lack of evidence.
Caveats aside, the dilemma when considering a patient who has a bona fide case of MPAL can be lessened by (1) gathering information, (2) considering pathophysiology, and (3) consulting the literature. The optimum information would include the patient’s age, past medical history/comorbidities, blast morphology (including cytochemistry), a complete immunophenotype, cytogenetics, and molecular studies. Older infirm patients with multiple comorbidities are not good candidates for standard ALL or AML induction therapy. The presence of Auer rods, degree of MPO+ on flow cytometry, and cytochemistry could be the basis for a therapeutic decision, albeit without any supporting data.
If they are rapidly available, cytogenetic studies could categorize the patient. Patients with MPAL and 11q23 rearrangement are considered a separate entity in the 2008 WHO schema, although their initial treatment may not be different from that for most MPAL patients. However, it is critical to define the Ph+ patient as rapidly as possible because such patients should have a tyrosine kinase inhibitor (TKI) added to their treatment. Finally, although the molecular biology has not been studied at any depth in MPAL, it makes sense to assess for the presence of mutations with prognostic and/or therapeutic relevance in leukemia and perhaps to rule out the presence of Ph-like signature, which could have eventual therapeutic implications in ALL.52 If nothing else, collecting the data may be retrospectively useful in learning about MPAL.
Because this disease is believed to emanate from a proximal, presumed long-lived stem cell in the hematopoietic hierarchy (high level of CD34 expression, capable of lineage switch or infidelity), one could surmise that chemotherapy alone would be insufficient to eradicate the disease. Ph+ ALL and MDS-associated and/or adverse chromosome AML are historically incurable without a stem-cell transplant; the same likely applies to MPAL. Moreover, the inclusion of more chemotherapeutic agents in up-front therapy used in an ALL or combined regimen would seem more logical than an AML regimen, especially the latter’s use of cytarabine, less useful against a slowly dividing primitive stem cell. Ideally, one could inhibit the gene product of a “founder” mutation present early in disease development and throughout the course. This serendipitous situation appears to be the case for Ph+ MPAL.
Ph+ and MLL rearranged MPAL
The only special cases within the MPAL WHO framework are patients with (9;22) or 11q23 cytogenetic abnormalities. Currently, treatment considerations for those with cytogenetic rearrangements at 11q23 are not different from those for MPAL with any non-Philadelphia cytogenetic abnormal or normal karyotype. However, the 11q23-rearranged patients should be considered for a pathophysiologically-based clinical trial if chemotherapy and alloSCT fail or if the patient is not a candidate for aggressive chemotherapy. Such therapy could include a histone-modifying–enzyme inhibitor or a bromodomain inhibitor based on the primary molecular abnormality53,54 or could target downstream activation of Hox genes via glycogen synthase kinase 3 or β-catenin inhibitors.55
Ph+ MPAL demand a specific approach involving the use of a TKI. This entity is usually a combination of B-lymphoid and myeloid markers; it accounts for about 25% of all MPAL.30 Essentially, all case series describing this entity mention the adverse prognosis engendered by this molecular lesion.29,40 However, in the TKI era, the situation may be changing.
One can reasonably look to the Ph+ ALL literature for guidance. Ph+ ALL was historically considered a poor prognostic entity, but prospective studies in which imatinib56,57 or dasatinib58 have been combined with standard multiagent chemotherapy depict a long-term DFS of 40% to 60%, approaching that seen with Ph– ALL in adults. Although Ph+ ALL patients achieving remission with TKI plus chemotherapy-based therapy conventionally should be consolidated with alloSCT if feasible, emerging data suggest that autologous transplant for patients with Ph+ ALL in remission59 and/or ongoing TKI as maintenance56 may be associated with long-term remissions, calling into question the obligate need for alloSCT in this MPAL subtype. For older adults, excellent short-term results have been obtained with dasatinib plus steroids and intrathecal chemotherpy60 ; one wonders about the need for aggressive chemotherapy even in younger patients given the potent antileukemic efficacy of TKIs. Therefore, we treat all MPAL t(9;22) patients with age-specific ALL chemotherapy in combination with a TKI (CALGB 9111,61 hyperCVAD,56 MRC-ECOG 299362 for middle-aged patients, and the Foa regimen60 for older patients) followed by alloSCT if feasible. In some pediatric and adult series, biphenotypic expression was reported to be associated with a high frequency of central nervous system (CNS) involvement at presentation44,49,50,63 ; thus we try to adhere to CNS-directed therapy according to the specific ALL protocol that is chosen. Whether to use highly effective pediatric-type chemotherapy64 plus TKI for patients under 40 with t(9;22) MPAL is unclear, although limited pediatric experience suggests this may be possible. A recent retrospective analysis compared characteristics and outcomes of 13 Ph+ MPAL patients with 27 patients with Ph+ ALL and demonstrated comparable CR rates among the 2 groups (100% vs 85%) as well as similar 5-year OS (55% vs 53%) and DFS (46% vs 42%).65
Non-Ph+ MPAL
What is the best approach for the non-t(9;22) MPAL patient? We treat with an ALL regimen and consolidate with an alloSCT if a donor is available. Most of the retrospective case series suggest that the CR rate is higher with ALL therapy or an ALL/AML combined regimen than with AML-type therapy. Matutes et al29 noted a CR rate of 85% compared with 41% for AML-type therapy. It is presumed that many of the patients who had morphologic AML (42%) received AML-type therapy; the inferior CR rate with this therapy may have been a manifestation of intrinsic resistance in this subset. Whether these “AML-like” patients would fare better with ALL-type therapy is unknown. Other studies, albeit with smaller patient numbers, showed similar findings regarding ALL-type vs AML-type CR rates: 75% vs 28%46 and 64% vs 33%, respectively.45
Although the preponderant thought has been to use ALL-type therapy, the situation is far from straightforward. Are there any patients in whom it makes sense to use AML-type therapy? Although we have tended to use 3+7 in MPAL patients who express MPO cytochemically, there is little support for this common sense approach. One prospective clinical trial used AML therapy in 7 MPAL patients who had >20% expression of MPO by flow but noted only 2 patients who achieved CR.46 We could find no data applying the use in MPO or Sudan black positivity to assign therapy. The use of combination AML+ALL regimens has some appeal (eg, the VAPA 10 approach66 ). These combined regimens vary among studies but generally add ALL-active agents such as steroids and vincristine to the AML anthracycline/cytarabine backbone. Remission rates with these combined regimens are largely comparable with those achieved with established ALL protocols,27,40,41,44-46 and some reports found these regimens to be rather toxic.40,44 In a few reports from adult and pediatric BAL series, high rates of remission with ALL-directed salvage therapy were reported after AML induction failure31,46 and vice versa.27
Emerging data suggest that pediatric-type regimens lead to a 50% to 60% 3-year survival in adults ages 18 to 40 with ALL,64,67 compared with a 30% to 40% rate with legacy regimens such as CALGB 9111 and hyperCVAD.61,68 Although MPALs were not specifically included in the few reports, it does make sense to use such therapy in patients up to age 40. Children with MPAL seem to fare better with ALL regimens, although they do have inferior outcome compared with other children with non-MPAL ALL.50 Although patients with MPAL who achieve CR and have an alloSCT are a select group, every study suggests superior outcomes in adult patients who receive alloSCT compared with those who receive only chemotherapy in the postremission setting.27,33 Moreover, alloSCT is better than chemo in essentially all high risk leukemias.69 For example,33 12 of 34 patients with ALAL underwent SCT. There was a 5-year OS rate of 70% compared with 19% for those who received chemotherapy only. Liu et al48 focused on 59 patients with ALAL who underwent alloSCT and noted a 5-year OS likelihood of 55% with an intensive preparative regimen and 24% with a standard preparation. Although using multiparameter flow cytometry to define minimal residual disease (MRD) in MPAL cases can be challenging, one could speculate that such patients who are clearly MRD– early in their course could be treated with consolidation chemotherapy rather than alloSCT in a similar fashion to Ph+ ALL that becomes molecularly negative after therapy.
Summary
As shown in Figure 3, a reasonable approach to a patient with MPAL is to first determine whether the disease is driven by BCR-ABL1. If so, age-appropriate ALL therapy plus a TKI followed by SCT is reasonable. If a patient is BCR-ABL1-negative, age-appropriate ALL therapy followed by SCT after remission is an acceptable strategy. Important areas for further study are: (1) whether the degree of MPO positivity by immunophenotype /cytochemistry should influence the choice of therapy, (2) whether the presence of myeloid-specific mutations or other genetic and molecular markers should be considered, (3) can the pathophysiology of 11q23 leukemia be successfully exploited, and (4) will alloSCT be needed for MPAL t(9;22) patients who respond very well to chemotherapy plus TKI?
The approach to therapy in patients with mixed-phenotype acute leukemia (MPAL). Targeted therapy based on a patient’s specific genetic profile should be considered in refractory/relapse patients (eg, MLL rearrangements, FLT3-ITD, IDH1, IDH2).
The approach to therapy in patients with mixed-phenotype acute leukemia (MPAL). Targeted therapy based on a patient’s specific genetic profile should be considered in refractory/relapse patients (eg, MLL rearrangements, FLT3-ITD, IDH1, IDH2).
Case presentation: part 2
Based on immunophenotype and cytogenetic information, the diagnosis of MPAL with t(9;22)(q34;q11.2);BCR-ABL1 was made. The patient was treated with a CALGB9111-type induction regimen plus dasatinib and promptly entered into CR; no immunophenotype-based MRD was noted, but BCR-ABL1 transcripts remained detectable. The patient received dasatinib and CNS prophylaxis followed by sibling-matched alloSCT with myeloablative conditioning. Three months after transplant, he remains in remission.
Acknowledgments
Dr Elizabeth Morgan from Brigham and Women’s Hospital, Department of Pathology, Harvard Medical School supplied and prepared the representative pathology images for Figure 1.
Authorship
Contribution: O.W. and R.M.S. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Richard M. Stone, Dana-Farber Cancer Institute, 450 Brookline Ave, Room D2053, Boston, MA 02115; e-mail: rstone@partners.org.