Key Points
SCT in first complete remission is associated with 69.5% 3-year overall survival in high-risk ALL adult patients treated with intensified pediatric-like protocol.
Poor early MRD response is a powerful tool to select patients who may benefit from SCT in first complete remission.
Abstract
Because a pediatric-inspired Group for Research on Adult Acute Lymphoblastic Leukemia (GRAALL) protocol yielded a markedly improved outcome in adults with Philadelphia chromosome–negative ALL, we aimed to reassess the role of allogeneic stem cell transplantation (SCT) in patients treated in the GRAALL-2003 and GRAALL-2005 trials. In all, 522 patients age 15 to 55 years old and presenting with at least 1 conventional high-risk factor were candidates for SCT in first complete remission. Among these, 282 (54%) received a transplant in first complete remission. At 3 years, posttransplant cumulative incidences of relapse, nonrelapse mortality, and relapse-free survival (RFS) were estimated at 19.5%, 15.5%, and 64.7%, respectively. Time-dependent analysis did not reveal a significant difference in RFS between SCT and no-SCT cohorts. However, SCT was associated with longer RFS in patients with postinduction minimal residual disease (MRD) ≥10−3 (hazard ratio, 0.40) but not in good MRD responders. In B-cell precursor ALL, SCT also benefitted patients with focal IKZF1 gene deletion (hazard ratio, 0.42). This article shows that poor early MRD response, in contrast to conventional ALL risk factors, is an excellent tool to identify patients who may benefit from allogeneic SCT in the context of intensified adult ALL therapy. Trial GRAALL-2003 was registered at www.clinicaltrials.gov as #NCT00222027; GRAALL-2005 was registered as #NCT00327678.
Medscape Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint providership of Medscape, LLC and the American Society of Hematology.
Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 2586.
Disclosures
The authors, Associate Editor Jacob M. Rowe, and CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, declare no competing financial interests.
Learning objectives
Describe outcomes of allogeneic stem cell transplantation (SCT) in first complete remission (CR1) among adults with high-risk acute lymphoblastic leukemia treated with intensified pediatric-like protocol, based on a review of clinical trial data.
Describe the use of poor early minimal residual disease as a tool to select patients who may benefit from SCT in CR1, based on a review of clinical trial data.
Describe the use of other risk factors as a tool to select patients who may benefit from SCT in CR1, based on a review of clinical trial data.
Release date: April 16, 2015; Expiration date: April 16, 2016
Introduction
Many studies have recently reported that adolescents and young adults with acute lymphoblastic leukemia (ALL) may benefit from pediatric rather than adult chemotherapy protocols, leading to evaluation of pediatric-like approaches in adults.1-7 Several groups are now using pediatric-inspired or even unmodified pediatric protocols in younger adults.8-12 In the first study of the Group for Research on Adult ALL (GRAALL), we showed that a pediatric-inspired protocol markedly improved the outcome of adult patients up to 60 years of age.13 In this new setting, we reassessed the role of allogeneic hematopoietic stem cell transplantation (SCT) in first complete remission (CR1) in patients treated in the GRAALL-2003 and GRAALL-2005 trials.
As in childhood ALL, minimal residual disease (MRD) levels significantly correlate with clinical outcomes in adult ALL trials.13-21 They might thus be considered as a stratification tool, with the option to offer allogeneic SCT to patients with persistent molecular disease.16,18,20,22 Conversely, novel genetic alterations originally described in children have also been reported to have an impact on the outcome of adult patients. In the B-cell precursor (BCP-ALL) subgroup, focal deletions of the IKZF1 gene, observed in up to 80% of Philadelphia chromosome (Ph)-positive ALL, have been associated with a poor outcome in adult patients with Ph-negative ALL.23-29 In the T-cell ALL (T-ALL) subgroup, we and others have found that mutations of the NOTCH1 pathway are associated with a better outcome that could be altered by the presence of additional NRAS and/or KRAS gene mutation or PTEN gene alteration.30-34 We have recently shown that MRD levels combined with these new genetic markers may predict relapse more efficiently than conventional risk factors.21 We thus sought to retrospectively assess the potential contributions of MRD and oncogenetic profiles to identify patients who may significantly benefit from SCT in CR1.
Patients and methods
GRAALL-2003 and GRAALL-2005 trials
The GRAALL-2003 and GRAALL-2005 trials were conducted between 2003 and 2011 in 70 centers in France, Belgium, and Switzerland. Protocols are provided in the supplemental Data, available online at the Blood Web site. Results of the GRAALL-2003 trial have already been reported.13 The subsequent GRAALL-2005 trial was very similar to the 2003 trial with the addition of randomized evaluation of hyperfractionated cyclophosphamide during induction and late intensification and rituximab during all phases of therapy in CD20+ BCP-ALL patients. Patients’ outcomes were last updated in January 2013. Per protocol, allogeneic SCT was offered in CR1 to patients age 55 years or younger who presented with at least 1 conventional ALL high-risk factor, as detailed below. Transplantation was planned after 3 or 6 blocks of consolidation, depending on the delay needed for donor identification. Most patients who received transplants from a related donor received 3 blocks of consolidation whereas those who received transplants from an unrelated donor mostly received 6 blocks. Informed consent was obtained from all patients at trial entry. Both trials were conducted in accordance with the declaration of Helsinki and approved by local and multicenter research ethical committees.
Risk factors used in the GRAALL-2003 and GRAALL-2005 trials
High-risk factors used to select patients eligible for allogeneic SCT in CR1 in these trials included (1) central nervous system involvement; (2) low hypodiploidy/near triploidy on karyotype and/or DNA index analysis; (3) early resistance to steroid prephase, defined as a peripheral blood (PB) blast cell count higher than 1.0 × 109/L after the prephase; (4) poor early bone marrow (BM) blast clearance, defined by morphologic BM blast cells of more than 5% after the first week of induction chemotherapy; (5) late CR, meaning that there was a need for the planned salvage course to reach CR; and (6) Ig/TCR MRD ≥10–2 after the first induction course in the GRAALL-2003 trial only. Additional factors were used in BCP-ALL patients, including white blood cell count (WBC) ≥30 × 109/L; MLL gene rearrangement, t[4;11] chromosomal translocation, and/or MLL-AF4 gene fusion, or other MLL rearrangement; and t(1;19) chromosomal translocation and/or E2A-PBX1 gene fusion. Two other factors were introduced in the GRAALL-2005 trial: complex karyotype and immature CD10– immunophenotype in BCP-ALL.
Study population
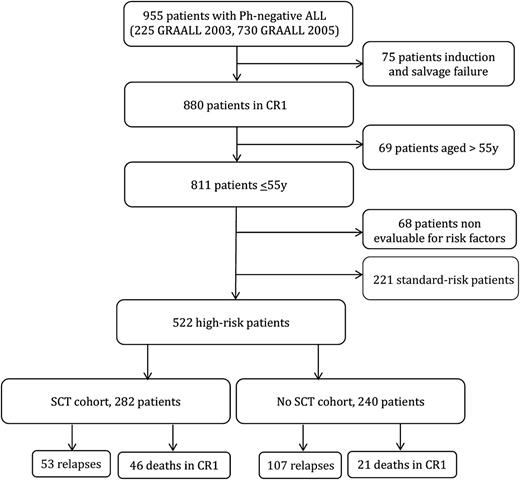
Overall, 955 patients age 15 to 60 years old with newly diagnosed Ph-negative ALL were enrolled in the GRAALL-2003 and GRAALL-2005 trials. Of the 880 patients (92%) who achieved CR, 811 were age 55 years or younger. Risk classification was available for 743 patients: 221 had standard-risk and 522 had high-risk ALL (Figure 1). The 5-year overall survival (OS) was 74% in the standard-risk group vs 58% in the high-risk group (supplemental Figure 1). These 522 high-risk patients were all candidates for SCT in CR1 and represented our study population.
Transplant modalities
In the GRAALL-2003 trial, SCT modalities depended on age, type of risk factor, and donor availability. Three SCT levels were defined, with potentially increasing toxicity. Patients with t(1;19) and/or E2A-PBX1 could receive only an HLA-identical sibling transplant up to the age of 45 years. Other eligible patients could receive an HLA-identical sibling transplant up to the age of 55 years or a 10/10 HLA-matched unrelated transplant up to the age of 45 years. Patients with MRD level ≥10–2 after the first consolidation course could receive either an HLA-identical sibling or a 10/10 HLA-matched unrelated transplant up to the age of 55 years. In the GRAALL-2005 trial, all eligible patients could receive an HLA-identical sibling or a 10/10 HLA-matched unrelated donor transplant until age 55 years, but transplant from a 9/10 HLA-mismatched donor was acceptable in patients with t(4;11)/MLL abnormality, low hypodiploidy/near triploidy, or late CR. The planned conditioning regimen for GRAALL-2003 and GRAALL-2005 was cyclophosphamide 60 mg/kg on 2 days and 12 Gy fractionated or 10 Gy unfractionated total body irradiation. Graft-versus-host disease prophylaxis consisted of cyclosporine and short-course methotrexate.
MRD analysis
MRD-level quantification was based on patient-specific Ig/TCR gene rearrangement monitoring, centrally performed on BM samples after the first induction course (MRD1; 6 weeks after induction initiation) and after the first 3 blocks of consolidation (MRD2; 12 weeks after induction initiation), as described.21 Briefly, DNA was extracted from BM samples, and its quality was assessed by albumin gene quantification by using standardized quantitative real-time polymerase chain reaction (qRT-PCR).35 Potential Ig/TCR targets were identified by using the standardized multiplex PCR established within the BIOMED-2/EuroClonality network.36 For each patient, preferably 2 independent Ig/TCR targets with a sensitivity of at least 10−4 and a quantitative range of 10−4 for at least 1 of the 2 targets were selected for MRD-level monitoring. All MRD data were assessed according to the guidelines developed within the EuroMRD group.35
New oncogenetic markers
BCP-ALL patients were centrally studied for focal IKZF1 gene deletion by using break point–specific multiplex PCR and multiplex ligation-probe assay, as described.21 T-ALL patients were centrally studied for the NOTCH1/FBXW7 gene mutation, NRAS and/or KRAS gene mutation, and PTEN gene alteration, as described.21 A T-ALL favorable genotype was defined by the presence of NOTCH1/FBXW7 mutation without NRAS and/or KRAS mutation or PTEN alteration, whereas the other combinations defined unfavorable genotypes.34
Statistical methods
Binary variable comparisons were performed by using Fisher’s exact test. Median comparisons were performed with the Mann-Whitney 2-sample test. The primary end point was relapse-free survival (RFS). Other end points were cumulative incidence of relapse (CIR), nonrelapse-related mortality (NRM), and OS from CR. RFS was defined as the time between achievement of hematologic CR and relapse or death in CR1, other patients being censored at the time of last contact. When evaluating CIR (events being hematologic relapses) and NRM (events being deaths in CR1), estimations took into account deaths in CR1 and relapses as competing events, respectively. Outcome comparisons were performed by Cox models.37 To evaluate the effect of SCT, we performed time-dependent analyses considering SCT in CR1 as a time-dependent event. Outcome data were estimated by the Mantel-Byar method38 and graphically illustrated by Simon-Makuch plots,39 t0 being the time of hematologic CR achievement. This time-dependent methodology allowed avoidance of the bias caused by the time to transplant, at least when analyzing CIR, NRM, and RFS. To avoid the bias related to early relapses when evaluating OS with this method, because patients must be alive and in CR1 to receive a transplant, we used a 45-day landmark period for OS comparisons. Outcome comparisons were performed by Andersen-Gill models.40 Interactions between SCT effect and covariables were tested by introducing interaction terms in multivariable models. We have recently reported that when MRD response and lineage-specific genetic markers were introduced in a multivariable risk model, only these 2 factors remained independent relapse predictors in patients treated with GRAALL protocols.21 Advanced age had an impact only on NRM.
On the basis of these observations, the 2 covariates retained for multivariable analyses were as follows: (1) the classical age, WBC, and resistance to steroid prephase covariates in the entire patient population; and (2) age, MRD1 response, lineage-specific genetic markers (ie, t(4;11)/MLL abnormality and focal IKZF1 gene deletion in BCP-ALL patients, high-risk genetic classifier in T-ALL patients) in the subsets of patients evaluated for MRD1 level and genetic markers. We also performed a landmark donor vs no-donor analysis (supplemental Data). Type 1 error was fixed at the 5% level. All tests were 2-tailed. Hazard ratios (HRs) were given with 95% confidence intervals (CIs). Statistical analysis was performed on the STATA/IC 12.1 software package (STATA, College Station, TX).
Results
Patient characteristics
Of the 522 study patients, 330 had a donor according to protocol criteria and 269 were actually transplanted in CR1. In addition, 13 patients received an unrelated cord blood (UCB) transplant in CR1, not planned by the protocol, leading to 282 patients (54%) in the SCT cohort. The reasons for 61 patients with a donor not receiving SCT in CR1 were early relapse (37 patients), investigator’s decision (11 patients), poor medical condition as a result of intercurrent adverse event (8 patients), early death in CR1 (2 patients), patient or donor refusal (2 patients), and unknown in the remaining patient. Characteristics of the 282 SCT and the 240 no-SCT patients are detailed in Table 1. There were no differences in baseline and early response characteristics between the two cohorts, except that more patients with t(4;11)/MLL abnormalities received SCT. Supplemental Table 1 shows a comparison between no-SCT patients with a donor and SCT patients or no-SCT patients without a donor.
Overall, SCT was performed from a matched sibling donor in 139 patients and an unrelated donor in 143 patients, including 92 fully 10/10 HLA-matched, 38 9/10 HLA-mismatched, and 13 with UCB. The source of hematopoietic stem cells was BM in 184 patients, PB in 85, and UCB in 13. Of note, 10 patients received reduced-intensity conditioning regimen and 17 patients were conditioned without total body irradiation. Median time between CR1 achievement and SCT was 106 days (range, 24 to 380 days) after a median number of 4 consolidation blocks (range, 0 to 6 consolidation blocks). As expected, this time was significantly shorter in patients who received SCT from a sibling donor compared with other patients (87 vs 123 days; P < .001). Accordingly, patients from the sibling donor subgroup received fewer pretransplant consolidation blocks (median, 3 vs 6 consolidation blocks; P < .001).
Outcome of patients who received allogeneic SCT in CR1
Among the 282 patients who were transplanted in CR1 and had a median posttransplant follow-up of 3.5 years, 53 patients relapsed and 89 patients died, including 46 deaths in CR1. At 3 years, posttransplant CIR and NRM were 19.5% (95% CI, 15% to 25%) and 15.5% (95% CI, 12% to 20%), respectively. This resulted in 3-year posttransplant RFS estimates of 64.7% (95% CI, 595 to 70%) and OS estimates of 69.5% (95% CI, 63% to 75%). As shown in Table 2, no difference was observed between BCP-ALL and T-ALL patients. The type of donor did not influence RFS (supplemental Figure 2). Advanced age and administration of a higher number of pretransplant consolidation cycles were both associated with a significantly higher posttransplant NRM (Table 2). Upon adjustment of donor type, age ≥45 years (HR, 1.9; P = .013) and more than 3 pretransplant consolidation cycles (HR, 2.0; P = .004) both remained independently associated with a higher NRM without significant impact on RFS.
Overall effect of SCT
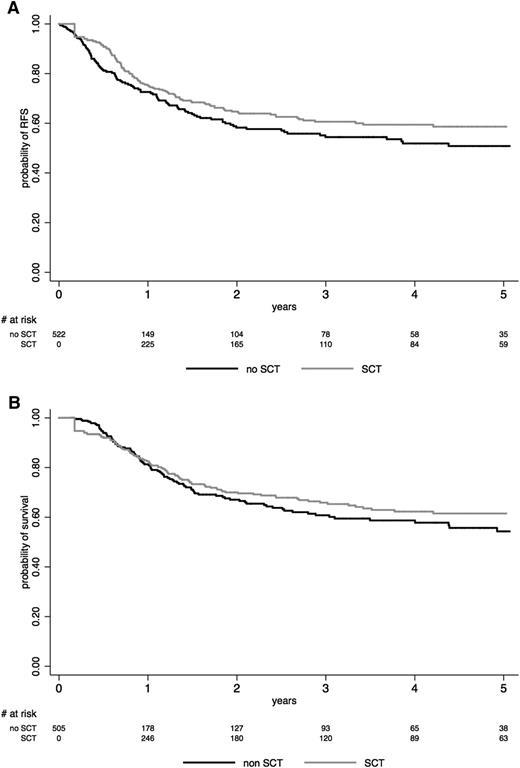
Among the 240 no-SCT patients, 107 relapsed and 107 died, including 21 deaths in CR1. When analyzing SCT in CR1 as a time-dependent event in the whole study population, RFS (HR, 0.80; 95% CI, 0.60 to 1.06; P = .12) and OS (HR, 0.76; 95% CI, 0.57 to 1.02; P = .069) were not significantly improved in the SCT cohort. The lower CIR (HR, 0.50; 95% CI, 0.35 to 0.70; P < .001) observed in the SCT cohort was counterbalanced by a higher NRM (HR, 1.46; 95% CI, 1.09 to 1.95; P = .011) (Table 3). This is illustrated in Figure 2, which shows Simon-Makuch plots for RFS and OS in both SCT and no-SCT cohorts.
Effect of SCT on (A) RFS and (B) OS. The effect of SCT in CR1 is represented on Simon-Makuch plots (SCT as a time-dependent covariable). Time t0 is CR achievement time. A 45-day landmark period was used for OS comparison. The black line represents the no-SCT patient cohort, and the gray line represents the SCT cohort.
Effect of SCT on (A) RFS and (B) OS. The effect of SCT in CR1 is represented on Simon-Makuch plots (SCT as a time-dependent covariable). Time t0 is CR achievement time. A 45-day landmark period was used for OS comparison. The black line represents the no-SCT patient cohort, and the gray line represents the SCT cohort.
As shown in Table 3, no significant effect of SCT on RFS was observed in patients younger or older than age 45 years. No effect was seen in patients with BCP-ALL or T-ALL when analyzed separately or in the different patient subsets defined by the prespecified baseline risk factors used in the protocols. Conversely, RFS and OS were significantly longer in the SCT than in the no-SCT cohort in patients who presented with morphologic poor early BM blast clearance or in late CR patients.
Effect of SCT according to early MRD level
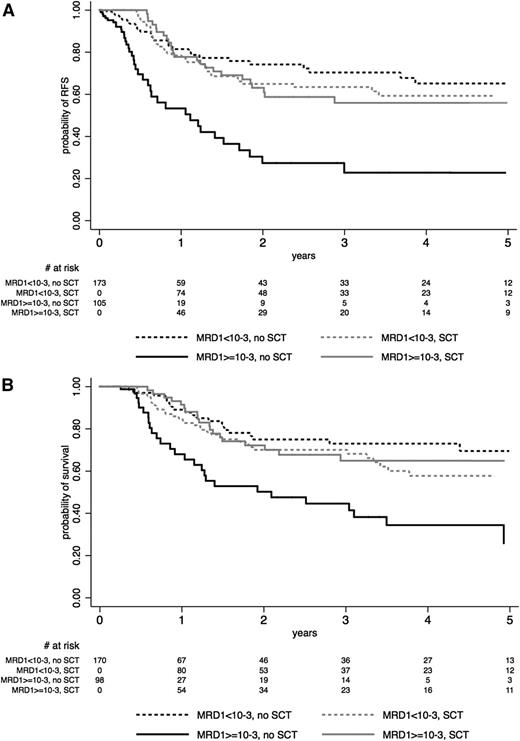
This latter observation led us to analyze the time-dependent effect of SCT according to postinduction MRD1 level (Table 4). Among the 522 study patients, 259 had MRD1 evaluation and 19 additional patients were not in CR at this time (late CR patients), meaning that 278 patients (53%) showed the presence of residual disease. As shown in supplemental Table 2, characteristics of these 278 patients did not differ from those of the 244 other patients. MRD1 level was ≥10−3 in 105 (37.7%) of those patients including the 19 late CR patients, with no difference between the SCT and the no-SCT cohorts (38.5% and 36.8%, respectively).
As detailed in Table 4, patients with MRD1 level <10−3 did not benefit from SCT in CR1 in term of RFS and OS, whereas those with a poor MRD1 response did benefit (Figure 3A-B). Tests for interactions were highly significant (RFS: P = .001; OS: P = .002; Table 4). Similar significant interactions were observed in the BCP-ALL and T-ALL subgroups when analyzed separately, even if the impact of SCT in poor MRD1 responders did not reach statistical significance in T-ALL patients (Table 4; illustrated for RFS in supplemental Figures 3 and 4). Supplemental Figure 5 illustrates that this interaction was also observed in the subset of 178 patients with poor early morphologic BM blast clearance, suggesting that MRD1 was a better tool than earlier blast clearance to define patients who may benefit from SCT. Similar results were found when excluding the 19 late CR patients from the analysis (data not shown).
Effect of SCT on (A) RFS and (B) OS, according to early MRD1 response. The effect of SCT in CR1 is represented on Simon-Makuch plots (SCT as a time-dependent covariable). Time t0 is CR achievement time. A 45-day landmark period was used for OS comparison. Solid lines represent patients who had MRD1 level ≥10−3 at t0 or late CR patients, and dashed lines represent patients who had MRD1 level <10−3 at t0. Black lines represent the no-SCT patient cohort, and gray lines represent the SCT cohort.
Effect of SCT on (A) RFS and (B) OS, according to early MRD1 response. The effect of SCT in CR1 is represented on Simon-Makuch plots (SCT as a time-dependent covariable). Time t0 is CR achievement time. A 45-day landmark period was used for OS comparison. Solid lines represent patients who had MRD1 level ≥10−3 at t0 or late CR patients, and dashed lines represent patients who had MRD1 level <10−3 at t0. Black lines represent the no-SCT patient cohort, and gray lines represent the SCT cohort.
Effect of SCT according to lineage-specific oncogenetic markers
In BCP-ALL patients, focal IKZF1 deletion was detected in 58 (28%) of 206 evaluable patients. Patients with IKZF1 deletion appeared to significantly benefit from SCT in terms of RFS and OS, whereas those without IKZF1 deletion did not (Table 4 and supplemental Figure 6 for RFS). As previously reported,21 the presence of IKZF1 deletion was strongly correlated with poor MRD1 response (60% vs 29% in poor MRD1 responders; P < .001). The role of SCT in the small subset of patients with MRD1 level <10−3 despite IKZF1 deletion (n = 18) could not be evaluated.
In T-ALL patients, a high-risk genetic profile according to our 4-gene classifier was found in 69 (54%) of 128 evaluable patients. Even if patient numbers were lower, RFS and OS appeared to be comparable in both SCT and no-SCT cohorts for both high-risk and low-risk genetic subsets, suggesting that high-risk profiles retained similar unfavorable prognostic value whether patients received SCT or not (Table 4 and supplemental Figure 7 for RFS).
Multivariable models
In the whole patient population, the time-dependent effect of SCT on RFS and OS still did not reach the significance level after adjustment on age, WBC, and resistance to steroid prephase in a multivariable model (RFS: HR, 0.79; 95% CI, 0.59 to 1.05; P = .10; OS: HR, 0.76; 95% CI, 0.57 to 1.03; P = .074). The same was observed when analyzing BCP-ALL and T-ALL patients separately. For BCP-ALL patients, HR was 0.77 (95% CI, 0.55 to 1.10; P = .15) for RFS and HR was 0.72 (95% CI, 0.51 to 1.03; P = .071) for OS. For T-ALL patients, HR was 0.83 (95% CI, 0.50 to 1.39; P = .47) for RFS and HR was 0.89 (95% CI, 0.51 to 1.56; P = .68) for OS. In the 278 patients studied for MRD1 levels, the significant interaction between MRD response and SCT effect was still observed after adjustment on the same covariates. For poor MRD1 responders, HR was 0.37 (95% CI, 0.20 to 0.69; P = .001) for RFS and HR was 0.41 (95% CI, 0.22 to 0.76; P = .005) for OS. For good MRD1 responders, HR was 1.38 (95% CI, 0.82 to 2.33; P = .23) for RFS and HR was 1.47 (95% CI, 0.85 to 2.54; P = .17) for OS. For RFS, Pinteraction = .001 and for OS, Pinteraction = .002.
Although we were limited by patient numbers, we performed multivariable analyses that included MRD1 response, lineage-specific genetic markers, age, and SCT as a time-dependent covariate in the 146 BCP-ALL and 76 T-ALL patients tested for MRD1 and genetic markers. In BCP-ALL patients, MRD1 response (RFS: P = .001; OS: P = .048), t(4;11)/MLL abnormality (RFS: P = .012; OS: P = .003), and IKZF1 deletion (RFS: P = .006; OS: P = .008) were the 3 factors that influenced RFS and OS. The effect of SCT remained apparent in patients with MRD1 ≥10−3 only (RFS: HR, 0.27; 95% CI, 0.12 to 0.60; P = .001; OS: HR, 0.32; 95% CI, 0.14 to 0.76; P = .01), with interactions between MRD1 response and SCT effect (RFS: P = .028; OS: P = 0.12). In T-ALL patients, MRD1 response (RFS: P = .019; OS: P = .038) and the 4-gene classifier (RFS: P = .009; OS: P = .086) were the two factors influencing RFS and OS. The effect of SCT in patients with MRD1 ≥10−3 did not reach the significance level, but interactions between MRD1 response and SCT effect were still observed (RFS: P = .061; OS: P = .034).
Discussion
In this study, we reassessed the role of allogeneic SCT in CR1 in adult patients with high-risk Ph-negative ALL treated with a pediatric-inspired protocol. We highlighted the value of early MRD quantification and IKZF1 gene deletion to discriminate patients who may currently benefit from SCT. To date, this study is the largest one that specifically investigated the relationship between early MRD response and SCT effect in a time-dependent manner.
Despite prior intensive 5-drug induction and dose-dense consolidation, posttransplant outcome was relatively good. With a 3-year NRM of 15.5% and a 3-year post-SCT survival of almost 70%, this outcome compared favorably with that of previous cohorts of ALL patients receiving myeloablative SCT in CR1.18,41-43 A higher NRM was nonetheless observed in patients age 45 years or older, which was consistent with the UKALL-12/ECOG E2993 trial and the recent donor vs no donor meta-analysis, both of which used the cutoff of age 35 years.44,45 Remarkably, patients who received transplants from related and unrelated donors displayed similar RFS. Similar results have been reported by other groups, which provided reassurance for considering unrelated as well as related donors for patients with an indication of SCT in CR1.42 Conversely, the excess of NRM observed in patients age 45 years or older encouraged us to prospectively evaluate the role of reduced-intensity conditioning SCT in these patients.
Because of the high proportion of SCT performed by using unrelated donors who are frequently not identified at the time of CR achievement, we used a time-dependent rather than a donor vs no-donor methodology to analyze the effect of SCT. We also performed a donor vs no-donor analysis (supplementary Table 3). We failed to find evidence of a beneficial SCT effect in the overall study population of patients defined as eligible for SCT by conventional risk factors. As is frequently observed, the lower CIR observed in the SCT cohort (HR, 0.50) was counterbalanced by a higher NRM resulting in comparable RFS (HR, 0.80). Furthermore, the analysis of the baseline high-risk factors did not identify any subset of patients for whom SCT significantly prolonged RFS. This is not totally surprising because those conventional factors were thought to be associated with a higher relapse risk rather than to interfere with transplant-related mortality. This nonetheless indicated that those factors were not the appropriate ones to define patients who could really benefit from SCT. This result appears to be conflicting with previous reports that show a superiority of allogeneic SCT over chemotherapy or autologous SCT.44-48 In several studies, however, allogeneic SCT was offered to all suitable patients with a donor, whereas it was offered only to selected high-risk patients in the GRAALL trials. Of importance, differences in the high-risk ALL definition, which could include age or not, might also have an impact or even yield opposite conclusions.44 Finally, improvement in the results of chemotherapy through the use of an intensified pediatric-like protocol is probably the main explanation for the lack of superiority of allogeneic SCT over chemotherapy in this article. The 50% RFS estimate at 5 years observed in our no-SCT cohort appears to be higher than that usually reported in similar patients after chemotherapy and compares favorably with results of allogeneic SCT in other studies.44-48
Nevertheless, we observed a significant effect of SCT in patients with poor early blast clearance or in late CR patients. This led us to evaluate the SCT effect according to early MRD response. In adult ALL, MRD response has been evaluated at various time points using different techniques and threshold cutoffs.16-18,20,49 Good MRD response was generally defined as an MRD level of <10−3 to 10−4 between weeks 5 and 10 and/or MRD level of <10−4 or not detectable between weeks 11 and 17. For logistical reasons, an earlier time point is more convenient for risk-oriented SCT decision. We thus used the 10−3 cutoff after the first induction course in this study. Using this cutoff at this time point, we showed that SCT erased the unfavorable prognostic impact of poor MRD response, whereas good MRD responders did not benefit from transplantation with significant interactions. Similar interactions were observed in BCP-ALL and T-ALL patients when analyzed separately. By using landmark analysis, the German Multicenter Study Group for Adult ALL (GMALL) has also reported that poor MRD responders receiving SCT displayed a better outcome than those treated with chemotherapy.18 Conversely, no superiority of SCT over chemotherapy was observed in patients with a poor early cytologic/MRD response in the Programa Español de Tratamientos en Hematología (PETHEMA) ALL-AR-03 trial, a result that could be hampered by a lower 5-year DFS after allogeneic transplant (32%) than expected in ALL in CR1.20
We also investigated the role of SCT in patient subsets defined by newly described oncogenetic markers. In BCP-ALL patients, the presence of poor-prognosis IKZF1 gene deletions was also predictive of a positive SCT effect but was strongly related to poor early MRD response. Conversely, in T-ALL patients, unfavorable genetic profiles were not predictive of any positive SCT effect and were not significantly related to poor early MRD response. From that point of view, it is very interesting to observe that the same T-cell genetic classification has recently been demonstrated as a strong outcome predictor in children and adults with T-cell lymphoblastic lymphoma, a thymic disease in which BM MRD evaluation is obviously of lower interest.50,51 For all these reasons, in the next GRAALL-2014 trial, we will base the decision of SCT in CR1 on early MRD response only.
In summary, we here report relatively good results associated with myeloablative SCT in CR1 in adults with Ph-negative high-risk ALL treated with a pediatric-inspired protocol. In this new setting, conventional baseline risk factors did not appear to satisfactorily identify SCT indications. Conversely, we confirm in a large prospective study that early MRD response is the best and maybe the unique tool to optimally select the patients who may currently benefit from SCT in CR1.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients and their families for participating in the Group for Research on Adult Acute Lymphoblastic Leukemia (GRAALL) trials and the GRAALL investigators (listed in the supplemental Data) for submitting clinical data and samples; Elodie Boucher, Nathalie Klein, Cyril Melot, Mathieu Sauvezie, and Francis Daniel for their help in data monitoring; Gérard Socié, Xavier Thomas, Elizabeth Macintyre, and Marie-Christine Béné for reviewing the manuscript; Nicole Raus, Ibrahim Yakoub-Agha, and the Société Française de Greffe de Moelle et de Thérapie Cellulaire for sharing data; and André Baruchel and Gérard Socié for their long-standing intellectual support.
This work was supported by grants 0200701, AOM 04144, and AOM 08106 from the Programme Hospitalier de Recherche Clinique, French Ministry of Health and Institut National du Cancer (H.D.), and by the Swiss State Secretariat for Education, Research and Innovations in Switzerland. Samples were collected and processed by the Assistance Publique-Hôpitaux de Paris Direction de Recherche Clinique Tumor Bank at Necker-Enfants Malades.
Written on behalf of the Group for Research on Adult Acute Lymphoblastic Leukemia (GRAALL). The GRAALL includes the former France-Belgium Group for Lymphoblastic Acute Leukemia in Adults, the French Western-Eastern Group for Lymphoblastic Acute Leukemia, and the Swiss Group for Clinical Cancer Research. GRAALL participating centers and investigators are listed in the supplemental Data.
Authorship
Contribution: N.D., A.H., S.M., X.T., C.G., Y.C., J.-Y.C., V.L., H.D., and N.I. conceived and designed the study; V.L. provided administrative support; N.D., A.H., S.M., R.T., X.T., P.C., S.N., V.C., J.-H.B., Y.H., M.E.-B., O.R., C.G., Y.C., D.B., J.-Y.C., H.D., and N.I. provided study materials or patients; N.D., A.H., S.M., K.B., V.A., V.L., H.D., and N.I. collected and assembled data; N.D., A.H., S.M., K.B., V.A., V.L., H.D., and N.I. analyzed and interpreted data; N.D., A.H., S.M., H.D., and N.I. helped write the manuscript; and all authors gave final approval to the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Norbert Ifrah, Service des Maladies du Sang, Centre Hospitalier Universitaire, 4 rue Larrey, 49933 Angers Cedex 9, France; e-mail: noifrah@chu-angers.fr; Hervé Dombret, Université Paris Diderot, Institut Universitaire d’Hématologie, EA-3518, Hôpital Saint-Louis, 1 avenue Claude Vellefaux, 75475 Paris Cedex 10, France; e-mail: herve.dombret@sls.aphp.fr.
References
Author notes
N.D. and A.H. contributed equally to this study.
H.D. and N.I. contributed equally to this study.