Key Points
Three-year follow-up of ibrutinib in CLL demonstrated continued activity with durable responses that improve in quality with extended treatment.
Toxicity diminished over time with respect to grade ≥3 cytopenias, fatigue, infections, and adverse events leading to discontinuation.
Abstract
Ibrutinib is an orally administered inhibitor of Bruton tyrosine kinase that antagonizes B-cell receptor, chemokine, and integrin-mediated signaling. In early-phase studies, ibrutinib demonstrated high response rates and prolonged progression-free survival (PFS) in chronic lymphocytic leukemia (CLL). The durable responses observed with ibrutinib relate in part to a modest toxicity profile that allows the majority of patients to receive continuous therapy for an extended period. We report on median 3-year follow-up of 132 patients with symptomatic treatment-naïve and relapsed/refractory CLL or small lymphocytic lymphoma. Longer treatment with ibrutinib was associated with improvement in response quality over time and durable remissions. Toxicity with longer follow-up diminished with respect to occurrence of grade 3 or greater cytopenias, fatigue, and infections. Progression remains uncommon, occurring primarily in some patients with relapsed del(17)(p13.1) and/or del(11)(q22.3) disease. Treatment-related lymphocytosis remains largely asymptomatic even when persisting >1 year and does not appear to alter longer-term PFS and overall survival compared with patients with partial response or better. Collectively, these data provide evidence that ibrutinib controls CLL disease manifestations and is well tolerated for an extended period; this information can help direct potential treatment options for different subgroups to diminish the long-term risk of relapse.
Introduction
Chronic lymphocytic leukemia (CLL), the most prevalent leukemia in adults, is defined by CD19, CD20, CD23, CD5, and variably low surface immunoglobulin expression.1 Genetic features identified in CLL that impact prognosis vary, with both recurrent cytogenetic abnormalities [del(13)(q14), trisomy 12, del(11)(q22.3), del(17)(p13.1), del(6)(q21)]2 and additional mutations (ATM,3,4 SF3B1,5 TP53,6 NOTCH1,7 XPO1,7 MYD88,7 and BIRC3).8 Patients with del(11q), del(17p), and TP53 mutations frequently progress earlier to symptomatic disease requiring therapy and have shorter response durations with traditional CLL therapies such as chemoimmunotherapy.6,9 Chemoimmunotherapy containing a CD20 antibody (rituximab,10 obinutuzumab,11 or ofatumumab12 ) with chemotherapy (fludarabine/cyclophosphamide or chlorambucil) generally prolongs survival in treatment-naïve (TN) patients with CLL, but is associated with significant toxicity (eg, immunosuppression and treatment-related neoplasms) and is usually not curative.13,14 Indeed, the majority of patients receiving chemoimmunotherapy relapse, particularly those with high-risk features, and experience a poor outcome. Although salvage treatment options are available, only allogeneic stem cell transplant represents a potential cure for relapsed patients. Challenges with this procedure include early and late morbidity and mortality, and it is not a practical option for many patients.15 Therefore, a major goal for CLL/small lymphocytic lymphoma (SLL) therapy is to identify treatments better targeted toward the malignant cell and its supportive microenvironment, thereby reducing toxicities experienced with chemotherapy.
Targeted therapy for leukemia is best exemplified by imatinib in chronic myeloid leukemia (CML), which has a disease-defining genetic abnormality: BCR-ABL1 rearrangement. Highly durable remissions were observed in TN patients with CML and in a lower frequency in previously treated patients.16,17 Although no single common genetic abnormality has been identified in CLL, the leukemia cell receives survival and proliferation signals from the microenvironment and the B-cell receptor (BCR) and is central to this interaction.18 This has prompted the utilization of kinase inhibitors (Bruton tyrosine kinase [BTK],19-21 spleen tyrosine kinase,22 and PI3 kinase23,24 ) directed toward the BCR pathway.19-21 Ibrutinib, a first-in-class, once-daily, orally administered selective covalent inhibitor of BTK, demonstrated promising preclinical and early clinical activity in CLL as part of ibrutinib’s first-in-human dose-escalation study in multiple B-cell lymphoid malignancies.19,21,25 A subsequent phase 1b/2 study (PCYC-1102) of ibrutinib in relapsed/refractory (R/R) and symptomatic older TN patients with CLL reported high response rates, sustained remissions, and acceptable toxicity.26,27 More recently, the phase 3 PCYC-1112 (RESONATE) trial in patients with previously treated CLL demonstrated a statistically significant (hazard ratio, 0.22; P < .001) 78% reduction in the rate of progression or death and a significant (hazard ratio, 0.43; P = .005) 57% reduction in the rate of death vs ofatumumab.28 Based on these results, single-agent ibrutinib (Imbruvica) received regular US Food and Drug Administration approval for patients with CLL who have received ≥1 prior therapy and for all patients with del(17p) CLL.29 As ibrutinib is a continuously administered oral once-daily therapy, data addressing the safety profile of ibrutinib over time, longer-term outcomes for those achieving partial response (PR) with lymphocytosis (PR-L), and efficacy in patient subgroups become increasingly relevant. We now report extended follow-up on all 132 patients enrolled in PCYC-1102 to determine response patterns, durability of remission across genetic and treatment groups, and extended term safety.
Patients and methods
Institutional review boards approved the study protocols at respective institutions, which were conducted according to the principles of the Declaration of Helsinki, the International Conference on Harmonization, and Good Clinical Practice guidelines. All patients provided written informed consent.
Patients and follow-up
Three-year safety was examined in patients from PCYC-1102 (NCT01105247) and the ongoing extension study PCYC-1103 (NCT01109069). The phase 1b/2 PCYC-1102 study included 85 patients with R/R CLL or SLL26 and 31 symptomatic TN patients with CLL/SLL aged ≥65 years.27 An additional 16 patients with CLL who had received ≥2 prior therapies (including purine analogs) and with otherwise similar eligibility criteria participated in a pharmacokinetic crossover evaluation examining the effect of food on ibrutinib exposure. Patients who completed a minimum of 6 treatment cycles with no evidence of disease progression could enroll in a long-term extension study (PCYC-1103) to continue ibrutinib therapy.
Treatment consisted of 420 or 840 mg/day ibrutinib administered orally until progressive disease or poor tolerance. Antimicrobial prophylaxis was permitted but not required. Patients receiving concomitant medications with weak/moderate CYP3A4/5 inhibitors were allowed to continue ibrutinib at investigator discretion with close monitoring. Although not originally specified in the protocol, early review of safety data revealed bleeding events in patients receiving concomitant warfarin, prompting restrictions against warfarin use in a subsequent amendment issued when 26 patients had discontinued treatment and 106 remained on ibrutinib, and alternative anticoagulation therapy was used. Guidelines to hold ibrutinib for 3 days before and after minor surgery and 7 days before and after major surgery were also implemented.
Safety and response assessments
The primary end point of this analysis was safety as assessed by the frequency and severity of grade ≥ 3 adverse events (AEs), serious AEs, and AEs requiring dose reduction or discontinuation. AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, v4.0. In PCYC-1102, all grade AEs were collected, whereas in PCYC-1103, only AEs grade ≥ 3, serious AEs, and those leading to dose modification or discontinuation were captured. AEs were reported as the worst grade experienced per patient. Long-term follow-up patients were seen by investigators every 3 months, at which time routine blood counts with differential, chemistries including liver function tests, physical examination, and response and toxicity assessments were performed.
Additional end points were overall response rate (ORR), complete response (CR), progression-free survival (PFS), and overall survival (OS). Response evaluations included computerized tomography radiologic examination every 6 months of the chest, abdomen, and pelvis and bone marrow biopsy to confirm CR as clinically appropriate.1 Patient response was evaluated based on International Workshop on Chronic Lymphocytic Leukemia 2008 guidelines.1 PR in all other disease parameters in the setting of persistent lymphocytosis was characterized as PR-L. Response in patients with SLL was evaluated based on the 2007 International Working Group criteria for non-Hodgkin lymphoma.30
Statistical analysis
Descriptive statistics, including mean, standard deviation, and median for continuous variables and proportions for discrete variables were used to summarize each of the defined cohorts. Because efficacy results for the 2 dose cohorts receiving 420 or 840 mg ibrutinib were similar, the data for all patients in each of the groups are reported together.26 All analyses were reported for all patients who received study drug. ORR with a 95% confidence interval (CI) was calculated. Kaplan-Meier methodology was used for time-to-event analyses. Patients were censored for PFS at the time of receiving new anticancer therapy or at last adequate disease assessment, whichever occurred earlier. No imputation of missing values was performed. Exploratory analyses investigating cytogenetic abnormalities del(17)(p13.1) and del(11)(q22.3) in association with PFS/OS used log-rank test without adjustment for multiplicity.
Results
Patient demographics
Baseline characteristics of 132 patients with CLL/SLL, including 31 TN patients age ≥65 years and 101 R/R patients are summarized in Table 1. Nearly half of all patients had Eastern Cooperative Group performance status (ECOG PS) 1, and 57% had Rai stage III to IV disease. Forty-six percent demonstrated bulky disease >5 cm at baseline. R/R patients had received a median of 4 prior therapies (range, 1-12), consisting primarily of chemotherapy and/or anti-CD20 antibodies. Patients with del(17p) also had a median of 4 prior therapies (range, 1-4). In the TN setting, the frequency of del(17p) and del(11q) was low (6% and 3%, respectively), reflective of patients at initial treatment, and 48% had unmutated IGHV status (<2% germ line). In the R/R cohort, 34% had del(17p), 35% had del(11q), and 78% had unmutated IGHV disease.
Concomitant medications
Concomitant medications of clinical interest received during the 3-year follow-up are summarized in supplemental Table 1 and included cytochrome p450 3A4 (CYP3A4) inhibitors (68%), antiplatelet agents (58%), intravenous immunoglobulin (45%), anticoagulants (22%), red blood cell and platelet transfusions in 20% and 7% respectively, and growth factors (14%).
Patient disposition and safety
Overall patient disposition is shown in Table 2. Median time on treatment was 30 months (range, 0.3-44 months) for TN patients, with 81% remaining on study treatment, and 23 months (range, 0.3-45 months) for R/R patients, with 53% remaining on study treatment. For TN and R/R patients, 81% and 46% received ibrutinib for >2 years, respectively.
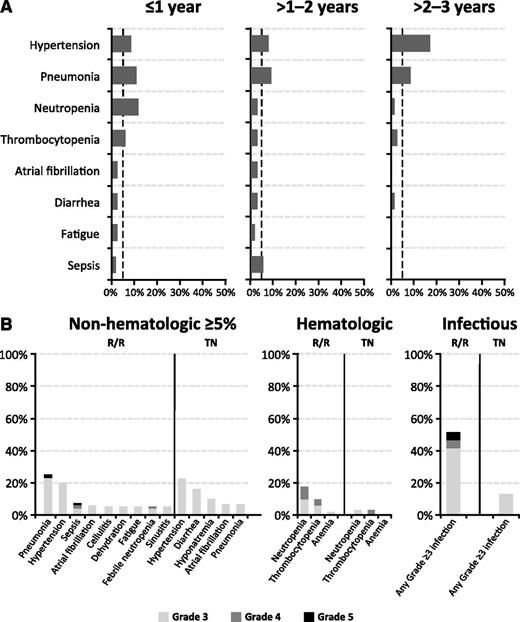
Table 3 summarizes grade ≥3 AEs. The most common AEs observed over the 3-year follow-up period were hypertension (23% TN, 20% R/R) and pneumonia (6% TN, 25% R/R). Neutropenia (3% TN, 18% R/R), thrombocytopenia (3% TN, 10% R/R), and pneumonia were more common in R/R patients, with a similar pattern observed for grade ≥3 infections (13% TN, 51% R/R). Of note, 2 cases of tumor lysis syndrome were reported, both in R/R patients: one (grade 4) occurred spontaneously prior to the first ibrutinib dose, and the second (grade 3) occurred in the setting of progressive disease 1 day after treatment discontinuation. For grade ≥ 3 AEs occurring in ≥5% of patients during years 1, 2, and 3 on therapy, the frequency of pneumonia, neutropenia, thrombocytopenia, diarrhea, and fatigue generally decreased over time, whereas the frequency of hypertension and atrial fibrillation appeared constant (Figure 1). Overall, TN patients generally experienced fewer grade ≥3 toxicities, particularly infectious and hematologic toxicities, compared with the previously treated cohort.
Serial assessment of adverse events over time. (A) Grade ≥3 AEs by time to event onset from first dose date. The dashed line denotes a 5% rate; x-axis maximum is 50%. (B) Frequency of grade ≥3 AEs by TN or R/R status.
Serial assessment of adverse events over time. (A) Grade ≥3 AEs by time to event onset from first dose date. The dashed line denotes a 5% rate; x-axis maximum is 50%. (B) Frequency of grade ≥3 AEs by TN or R/R status.
Dose reductions due to AEs were reported for 13 patients and occurred primarily during the first year of treatment. The only AE leading to dose modifications in ≥1 patient was diarrhea (n = 2). The primary reasons for discontinuing therapy for all patients included disease progression in 22 patients (17%) and AEs in 17 patients (13%). For AEs requiring treatment discontinuation, only 4 were possibly related to ibrutinib treatment (grade 3 subdural hematoma in a R/R patient, grade 3 influenza in an R/R patient, grade 3 pruritic rash in a TN patient, and grade 3 fatigue in a TN patient), and all resolved. Discontinuations due to AEs predominantly occurred within the first year of treatment (11/132 patients [8% overall]) and declined during the second (4/103 [4%] patients) and third (2/71 [2%] patients) years of follow-up. Expanded information on the presentation and management of select toxicities are provided below.
Diarrhea
Diarrhea, observed in 77 (58%) patients (68% TN and 55% R/R), was the most frequent any-grade AE. The majority of events were mild in severity. The median number of diarrhea events per patient was 1 (range, 1-6), and the median duration of diarrhea episode was 20 days (range, 1-779 days). Overall, 80 episodes (71%) resolved during study follow-up. Eight patients experienced 12 events of grade 3 diarrhea, with 3 patients having ≥1 occurrence. There were no grade 4 or 5 events. Most grade 3 events were transient, with 5 events extending beyond 5 days. The majority of events resolved. Diarrhea was generally self-limited, typically resolving without additional therapy. Some patients received symptomatic management with antimotility agents, which was generally successful in treating diarrhea once infectious etiologies were ruled out. One grade 3 event led to study treatment discontinuation. Two patients had diarrhea events managed with ibrutinib dose reductions. Three events had confounding factors of Clostridium difficile colitis (n = 2; one with grade 3 diarrhea and one with grade 2 diarrhea) and hemorrhagic colitis (n = 1; with grade 3 diarrhea); all 3 events resulted in treatment delay. Onset, grade, and duration of diarrhea events are summarized in supplemental Figure 1.
Infections
The frequency of grade ≥ 3 infections was higher in R/R patients (51%) vs TN patients (13%), respectively, including pneumonia (25% vs 6%), sepsis (7% vs 0%), cellulitis (5% vs 0%), sinusitis (5% vs 0%), and bacteremia (4% vs 0%; Table 3). Types of infections and pathogens identified are summarized in supplemental Table 2. Grade ≥3 infections were observed more frequently during the first year of therapy: R/R (36%, 32%, and 24%) and TN (10%, 8%, and 4%) for years 1, 2, and 3, respectively. Intravenous immunoglobulin supplementation was administered to 5 (16%) TN patients and 54 (53%) R/R patients, which was continued as supportive care that was started before enrollment on the study. Development of viral infections, particularly varicella zoster, can be problematic in CLL patients receiving fludarabine therapy.31 Antiviral prophylaxis was used at some point during the 3-year follow-up in 85 R/R and 10 TN patients; of these patients, 71 R/R and 3 TN patients were continuing antiviral prophylaxis initiated before starting ibrutinib. No TN patients and 5 R/R patients experienced zoster reactivation during the 3-year follow-up (including 1 grade 3).
Cytopenias
Eighteen R/R patients (18%) and 1 TN patient (3%) developed grade ≥3 neutropenia, and 5 R/R patients experienced febrile neutropenia (5%) during the 3-year follow-up. At some point during extended follow-up, 18 R/R patients received growth factor support for neutropenia (filgrastim, pegfilgrastim, or sargramostim) and 3 patients (1 TN and 2 R/R) received erythropoietin stimulating agents. Eleven patients (10 R/R [10%] and 1 TN [1%]) had grade ≥ 3 thrombocytopenia. One grade 3 and one grade 4 neutropenia and one grade 2 anemia led to a dose reduction; no events of thrombocytopenia required dose reduction. No cytopenias led to treatment discontinuation.
Bleeding
The rate of bleeding AEs was 61% overall, including 48% grade 1, 5% grade 2, 7% grade 3, and 1% grade 5. The most commonly reported terms that contributed to bleeding rate were contusion and petechiae. Of the 132 patients, the use of aspirin (acetylsalicylic acid) was common (52% TN and 28% RR), with anticoagulant use reported in 10% and 26%, respectively (supplemental Table 1). Throughout the 3-year follow-up, 10 patients experienced a major hemorrhage. Bleeding events resulted in treatment discontinuation in 3 patients (subdural hematoma in 2 and gastrointestinal hemorrhage in 1). The only grade 5 bleeding event occurred in a patient who died with a bilateral subdural hematoma that occurred ∼1 month after ibrutinib discontinuation for progressive disease in the setting of multiorgan failure (liver and renal) and coagulopathy (likely consumptive).
Response
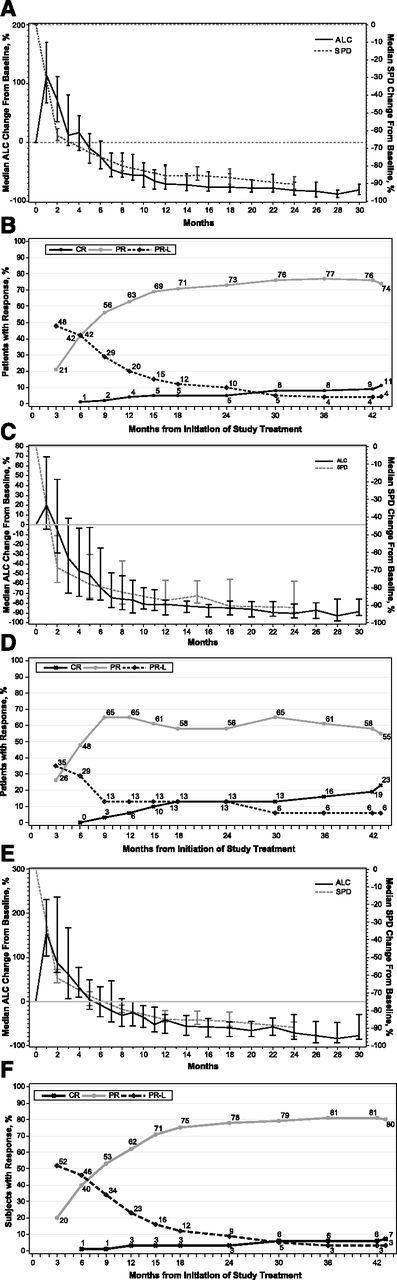
The overall best response to ibrutinib therapy by investigator assessment is summarized in Table 4 and supplemental Table 3. Previously untreated patients showed an ORR of 84%, with 23% attaining CR, 55% PR, and 6% PR-L. Although treatment with ibrutinib continued until progression, it is notable that one patient who achieved PR and then stopped ibrutinib for toxicity (rash) was followed for another 17 months before initiating subsequent anticancer therapy. For the R/R cohort, a 90% ORR was observed, with 7% of patients achieving CR, 80% PR, and 3% PR-L. Five of 6 patients with prior idelalisib achieved PR (83%), with the remaining patient experiencing fatal cryptoccocal pneumonia at 0.7 months. Among all treated patients, median time to initial response was 1.9 months (range, 1.4-12.2 months), which was the approximate timing of the first protocol-required response assessment (2 months), whereas median time to best response was 7.4 months (range, 1.7-42.5 months), with CR being achieved as late as 42.5 months into ibrutinib therapy. Response quality evolves over time, as the median time to CR was 21.2 months (range, 4.6-42.5 months). Notably, with extended follow-up, 94% of patients who achieved PR-L converted to a deeper response (CR or PR; Figure 2). Following initial publication of the results,26,27 an independent review was performed to provide confirmation of response assessments by trial investigators. Concordance rates of responders to nonresponders were 97% for TN patients and 88% for R/R patients.
Response to ibrutinib over time. (A) Median percent change from baseline in the ALC and the sum of the products of lymph node diameters (SPD) in all patients, showing 95% confidence intervals for all patients. (B) Curves for cumulative best response (CR, PR, and PR-L) for all patients (N = 132). Results for TN patients (n = 31) are shown in C and D. Results for R/R patients (n = 101) are shown in E and F.
Response to ibrutinib over time. (A) Median percent change from baseline in the ALC and the sum of the products of lymph node diameters (SPD) in all patients, showing 95% confidence intervals for all patients. (B) Curves for cumulative best response (CR, PR, and PR-L) for all patients (N = 132). Results for TN patients (n = 31) are shown in C and D. Results for R/R patients (n = 101) are shown in E and F.
Improvement in cytopenias
The median baseline hemoglobin level was 122 g/L (range, 77-157 g/L) for TN patients and 115 g/L (range, 62-176 g/L) for R/R patients, median baseline platelet counts were 113 × 109/L (range, 32-217 × 109/L) and 105 × 109/L (range, 2-310 × 109/L), and median baseline absolute neutrophil counts were 3.9 × 109/L (range, 0-19.4 × 109/L) and 2.5 × 109/L (range, 0-19 × 109/L) for TN and R/R patients, respectively. Among patients with baseline anemia or thrombocytopenia, hemoglobin levels and platelet counts improved over time (Figure 3). At the last visit on treatment, median hemoglobin levels were 136 g/L (range, 84-162 g/L) and 133 g/L (range, 79-173 g/L), median platelet counts were 148 × 109/L (range, 33-452 × 109/L) and 124 × 109/L (range, 7-643 × 109/L), and median neutrophil counts were 3.8 × 109/L (range, 1.2-11.4 × 109/L) and 3.6 × 109/L (range, 0.1-15.3 × 109/L) for TN and R/R patients, respectively.
Hemoglobin levels and platelet counts in all treated patients with baseline anemias and thrombocytopenias, respectively.
Hemoglobin levels and platelet counts in all treated patients with baseline anemias and thrombocytopenias, respectively.
Lymphocytosis
The majority of patients (96 [73%]) experienced an increase in peripheral blood lymphocyte count that peaked at a median of 4 weeks (range, 0.6-136 weeks), followed by a decline that varied based on genetic characteristics, including a more protracted decline frequently observed in lower-risk patients with del(13)(q14) or mutated IGHV status, as previously reported.32,33 Supplemental Table 4 shows the starting and peak absolute lymphocyte counts (ALCs). Of 18 patients (14%) with baseline ALC >100 × 109/L, 4 developed ALC ≥ 400 × 109/L. Of the 16 patients (12%) with baseline ALC 50 to 100 × 109/L and the 98 patients (74%) with baseline ALC <50 × 109/L, none developed ALC ≥400 × 109/L. This lymphocytosis was largely asymptomatic and resolved with continued ibrutinib therapy (median time to resolution, 19 weeks [range, 1-124 weeks]).
PFS
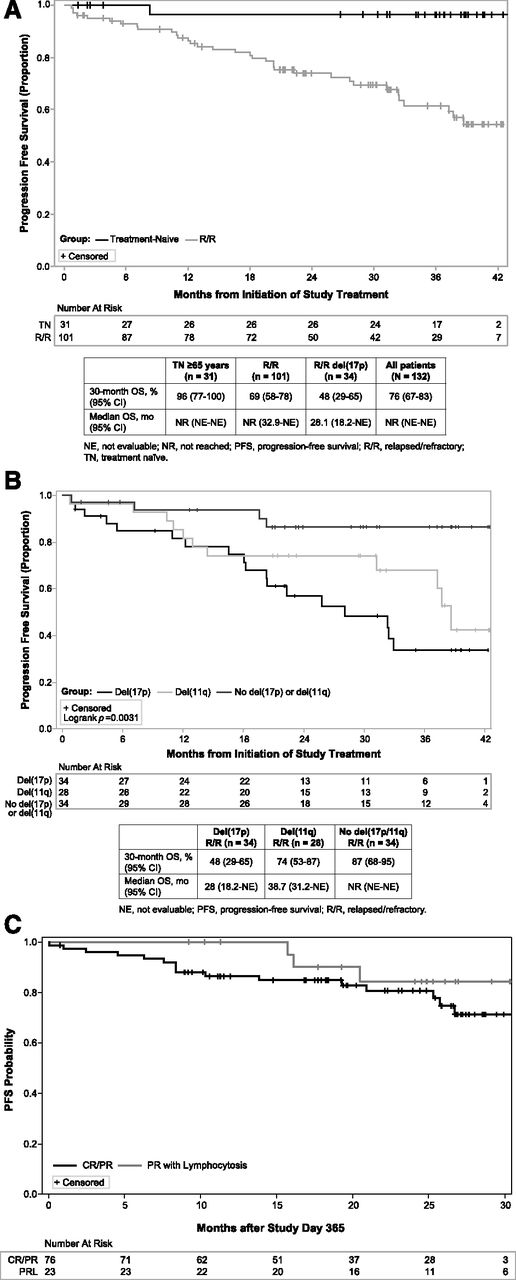
With a median time on study of 35.2 months, median PFS was not reached for all TN or RR patients. In patients with TN CLL/SLL as depicted in Figure 4A, the estimated PFS rate was 96% (95% CI, 76.5-99.5%) at 30 months. The only patient with progression at 8 months had a del(17p) abnormality, as previously described.27 In previously treated patients with CLL/SLL as depicted in Figure 4A, the estimated PFS at 30 months was 69% (95% CI, 58-78%). As shown in Figure 4B, PFS with ibrutinib varies by interphase cytogenetic abnormality (P = .003), with del(17p) patients having a 30-month estimated PFS rate of 48% (95% CI, 29-65%), which is less than the 74% rate (95% CI, 53-87%) observed for del(11q) and the 87% rate (95% CI, 68-95%) observed when neither of these aberrations is present. Of the 25 patients who progressed, 23 had ≥1 aberration: 14 del(17p) and 10 del(11q). Patients who experienced lymphocytosis experienced outcomes similar to those without lymphocytosis as evidenced by a landmark PFS analysis among patients with persistent lymphocytosis and PR-L vs PR or better (Figure 4C) at 1 year. Of the 25 patients with disease progression during extended follow-up, 8 had Richter’s transformation (including the 1 TN patient27 ).
PFS. (A) Kaplan-Meier curves of PFS in TN and R/R patients. (B) Kaplan-Meier curves of PFS in patients with del(17p) or del(11q) or without del(17p) or del(11q). (C) Kaplan-Meier curves of PFS from day 365 in patients who achieved CR and PR or PR-L within the first 364 days on study.
PFS. (A) Kaplan-Meier curves of PFS in TN and R/R patients. (B) Kaplan-Meier curves of PFS in patients with del(17p) or del(11q) or without del(17p) or del(11q). (C) Kaplan-Meier curves of PFS from day 365 in patients who achieved CR and PR or PR-L within the first 364 days on study.
OS
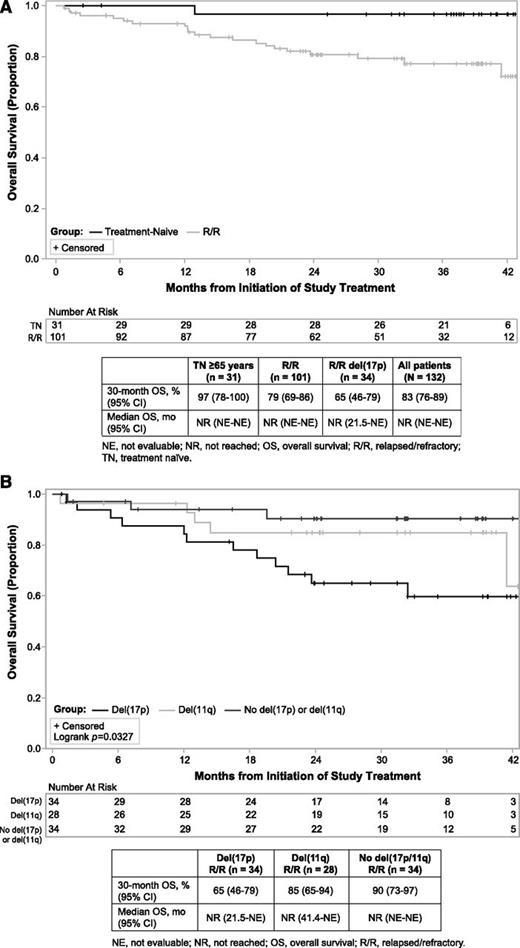
With 3-year follow-up, median OS was not reached for all patients. For patients with TN CLL, the estimated 30-month OS rate was 97% (95% CI, 78-99.5%; Figure 5A). In patients with previously treated CLL, the 30-month OS was 79% (95% CI, 69-86%; Figure 5A). Similar to PFS in patients with heavily pretreated disease, OS with ibrutinib varies by interphase cytogenetic abnormality (P = .0327), with del(17p) patients having a 30-month estimated OS rate of 65% (95% CI, 46-79%), which is shorter than the 85% rate (95% CI, 65-94%) observed for del(11q) and the 90% rate (95% CI, 73-97%) observed when neither aberration is present (Figure 5B).
OS. (A) Kaplan-Meier curves of OS in TN and R/R patients. (B) Kaplan-Meier curves of OS in patients with del(17p) or del(11q) or without del(17p) or del(11q).
OS. (A) Kaplan-Meier curves of OS in TN and R/R patients. (B) Kaplan-Meier curves of OS in patients with del(17p) or del(11q) or without del(17p) or del(11q).
Discussion
We described extended follow-up of single-agent, once-daily ibrutinib in patients aged ≥ 65 years with TN CLL and in patients with previously treated CLL from the PCYC-1102 and the ongoing long-term extension study PCYC-1103. These data continue to demonstrate that ibrutinib promotes a high response rate that improves in quality with time, leading to durable remissions in all subsets of CLL patients. Unlike chemotherapy and chemoimmunotherapy, which are given for a defined period because of limited tolerability, toxicities observed with continuous ibrutinib dosing are modest, allowing most patients to remain on ibrutinib for an extended period to maximize response (Figure 2B).
These data demonstrate that with continuous ibrutinib therapy, responses improve in quality and frequency over time. The CR rate continues to improve, now reported in 23% and 7% of TN and R/R patients, respectively, with a median time to CR of 21 months and best responses achieved as late as 42.5 months of treatment. Both the CR and 30-month PFS rate (96% vs 69%, respectively) were higher in TN patients compared with R/R patients. Notably, with 3 years of follow-up, 81% of TN patients continue daily ibrutinib. There have been no further relapses for over 2 years. This gives some suggestion that the best outcomes may be seen when ibrutinib is administered as first-line rather than salvage therapy. However, the low number of genomic high-risk patients in the untreated group prevents firm conclusions on this matter. Furthermore, toxicity leading to ibrutinib discontinuation and toxicities such as cytopenias, fatigue, diarrhea, and infections diminished with continued treatment. However, as observed with other CLL therapies, genomic aberrations in this heavily pretreated population appear to affect PFS. Nonetheless, the PFS (median, 28.1 months) observed in the 34-patient cohort of relapsed patients with del(17p), with a median of 4 prior therapies, surpasses that observed with any therapy published for this group, including those receiving first-line treatment.10,34-36 Ibrutinib represents a significant advance in the treatment options available for these patients. Collectively, 3-year follow-up of this trial supports the potentially transformative role ibrutinib may have in the future of CLL therapy.
The occurrence of early lymphocytosis, a class effect observed with agents that target BCR signaling, including inhibitors of BTK,25 spleen tyrosine kinase,37 and PI3 kinase,38 initially raised concerns that this could be a manifestation of disease progression. However, groups of experts, including International Workshop on Chronic Lymphocytic Leukemia authors, the National Comprehensive Cancer Network, and a workshop on endpoints sponsored by the Lymphoma Research Foundation, convened to clarify that patients with CLL treated with BCR-inhibiting agents who experience peripheral lymphocytosis in the setting of improvement in other parameters are not experiencing treatment failure (ie, stable disease or progression).1,28,39,40 Although this relative increase in peripheral lymphocytosis can be pronounced in some patients, particularly in those with lower baseline lymphocyte counts, it is generally asymptomatic. Further, lymphocytosis typically occurs early during treatment and in the setting of concurrent improvement in lymph node, spleen, and/or cytopenias and subsequently resolves in the majority of patients (96%) with continued treatment. These findings confirm with additional follow-up the work of Woyach at al,33 showing that patients with persistent lymphocytosis at 1 year have similar to improved PFS and OS compared with those with PR or CR. The durable remissions observed in patients with PR-L may render treatment with additional agents such as rituximab or ofatumumab for the sole purpose of eliminating peripheral lymphocytosis unnecessary. Randomized trials are ongoing to formally assess the long-term benefit of combining ibrutinib with anti-CD20 monoclonal antibodies.
Infectious complications observed with ibrutinib are considerably fewer in TN compared with R/R patients and appear to occur more frequently during the early part of treatment, diminishing as patients continue ibrutinib. Reasons for the decrease in infectious morbidity over time are potentially related to several factors, including early responses and disease control (potentially due to the reduction of immunosuppressive T cells), extended time from last chemotherapy (in the case of R/R patients), and the immune-modulating potential of ibrutinib through inhibition of interleukin-associated T-cell kinase, which promotes T helper cell type 1 CD4 T-cell outgrowth and, in 2 animal models of infection, diminishes infection morbidity.41 With continuous BTK inhibition by ibrutinib, a subset of both TN and R/R CLL patients received intravenous immunoglobulin, and maintenance or restoration of IgA levels was observed. Reasons for immunoglobulin level stabilization or improvement are uncertain but may relate to lack of BTK in normal plasma cells that allows acquired humoral memory to function in the setting of sustained BTK inhibition with ibrutinib. Further study of this is warranted.
Finally, extended follow-up demonstrates that even in multiply relapsed patients, those without del(17p) or del(11q) experience prolonged PFS (87% estimated at 30 months), whereas a subset of these heavily pretreated patients, in particular those with del(17p), are predisposed to relapse. Still, median PFS (28 months) in this heavily pretreated population (median 4 prior lines) with del(17p) CLL compares favorably to that of even TN patients with CLL and del(17p) receiving fludarabine, cyclophosphamide, and rituximab (FCR)10 or alemtuzumab alone35 or in combination with steroids,34 with a median PFS of 11 to 18 months reported for frontline therapy. It is possible that less heavily pretreated patients with del(17p) may fare even better. Further, a more recent study of single-agent ibrutinib in TN and R/R CLL patients conducted at the National Heart, Lung, and Blood Institute showed similar responses in patients with and without del(17p).42 CLL progression occurs infrequently in ibrutinib-treated patients and almost exclusively in those with high-risk cytogenetic abnormalities.43 Evaluation of combination strategies in this poor-risk group of patients with high-risk genetic abnormalities is ongoing. Extended treatment with single-agent ibrutinib earlier in the course of CLL at the time of initial treatment before these abnormalities develop could improve patient outcomes. To address this important question, randomized trials comparing ibrutinib with standard first-line therapies are ongoing including RESONATE-2 (NCT01722487), ALLIANCE (NCT01886872), E1912 (NCT02048813), and the United Kingdom-based CLL10.
Presented in part at the 2014 annual meeting of the American Society of Clinical Oncology, May 31-June 4, 2014, Chicago, IL.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the investigators and coordinators at each of the clinical study sites and the patients who participated in these studies and their families. The authors thank Pharmacyclics employees who contributed to the design and implementation of these trials, Anh Tran for study management, Stephan Chan and the Pharmacyclics biometrics team for providing support, and Robert Rydzewski, CMPP, for editorial assistance with the manuscript after the first draft that was generated by J.C.B.
This work was supported by the Specialized Center of Research program of the Leukemia and Lymphoma Society, National Institutes of Health, National Cancer Institute grants K12 CA133250, P50-CA140158, P01 CA95426, P01 CA8153, and P01 CA101956, and The D. Warren Brown Foundation.
Authorship
Contribution: J.C.B., R.R.F., A.J.J., D.F.J., and S.O. designed the study; J.C.B., R.R.F., S.E.C., J.A.B., K.A.B., M.C., W.G.W., J.A.J., A.J.J., and Y.S. collected data; J.C.B., W.Z., N.A.H., E.B., C.Z., D.F.J., and S.O. analyzed and interpreted data; C.Z. performed statistical analysis; and all authors were involved in manuscript preparation and approved the final version of the manuscript.
Conflict-of-interest disclosure: J.C.B. received research funding from Pharmacyclics. R.R.F. is a consultant and on the speakers bureau for Pharmacyclics. S.E.C. has received research funding and honoraria from Janssen. J.A.B. is a consultant for and has received honoraria from Pharmacyclics. K.A.B. received research funding from Pharmacyclics. M.C. received research funding from Pharmacyclics, GSK, and Gilead and is a consultant for Amgen and Pfizer. W.G.W. has received research funding from and is a consultant for Pharmacyclics. Y.S, E.B., C.Z., and D.F.J. are employees of Pharmacyclics. S.O. received research support and/or is a consultant for Pharmacyclics, Amgen, Celgene, Emergent, Genentech, Gilead Sciences, GSK, Infinity, MorphoSys, Spectrum, Acerta, and TG Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: John C. Byrd, OSU CCC Bldg, Room 455B, 10 West 12th Ave, Columbus, OH 43210; e-mail: john.byrd@osumc.edu.