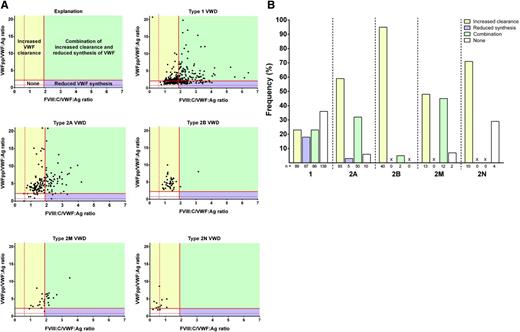
In this issue of Blood,Sanders and coworkers define the pathophysiology of types 1, 2, and 3 von Willebrand disease (VWD) in the Willebrand in the Netherlands (WiN) study by using the ratios of von Willebrand factor propeptide (VWFpp) or factor VIII activity to VWF antigen.1
Pathophysiological mechanisms per type of VWD. (A) FVIII:C/VWF:Ag vs VWFpp/VWF:Ag for types 1, 2A, 2B, 2M, and 2N VWD. Potential mechanisms include increased clearance (yellow), reduced synthesis (purple), combination of both (green), and other mechanisms (white). (B) Proportion of VWF patients with increased clearance (yellow), reduced synthesis (purple), combination of both (green), and other (white). See Figure 2A-B in the article by Sanders et al that begins on page 3006.
Pathophysiological mechanisms per type of VWD. (A) FVIII:C/VWF:Ag vs VWFpp/VWF:Ag for types 1, 2A, 2B, 2M, and 2N VWD. Potential mechanisms include increased clearance (yellow), reduced synthesis (purple), combination of both (green), and other mechanisms (white). (B) Proportion of VWF patients with increased clearance (yellow), reduced synthesis (purple), combination of both (green), and other (white). See Figure 2A-B in the article by Sanders et al that begins on page 3006.
Von Willebrand factor (VWF) is synthesized in endothelial cells and megakaryocytes as pre-proVWF consisting of a 22–amino acid (aa) signal peptide, 741-aa VWFpp, and 2050-aa mature VWF molecule. After extensive intracellular modifications, VWF and VWFpp are packaged in endothelial cell Weibel-Palade bodies or platelet α-granules for regulated secretion. In plasma, VWFpp and VWF circulate independently of one another, with half-lives of 2 to 3 hours and 8 to 12 hours, respectively.2 The ratio of VWFpp to VWF antigen (VWF:Ag) can be used to assess the rates of synthesis, secretion, and clearance of VWF.2-4 The ratio of FVIII coagulant activity (FVIII:C) to VWF:Ag has been used in a similar manner.4 An increased ratio of VWFpp to VWF:Ag is used to identify patients with acquired von Willebrand syndrome or reduced VWF survival (type 1C VWD).3,5
Discriminating between VWD subtypes is often difficult, particularly when VWF levels are extremely low. Sanders and colleagues report a cross-sectional assessment of the utility of VWFpp/VWF:Ag and FVIII:C/VWF:Ag ratios in the diagnosis of types 1, 2, and 3 VWD.1 By plotting FVIII:C/VWF:Ag vs VWFpp/VWF:Ag for VWD subtypes, the pathologic mechanism may be predicted in a rather straightforward manner (see figure, panel A). An increased ratio of VWFpp/VWF:Ag defines reduced survival of VWF, and an increased ratio of FVIII:C/VWF:Ag suggests reduced VWF synthesis. The pathophysiology within VWD subtypes is diverse, including primarily increased VWF clearance, reduced VWF synthesis, a combination of increased clearance/reduced synthesis, and other undefined mechanisms (see figure, panel A). The relative contribution of each mechanism to phenotype is variable among subtypes (see figure, panel B). However, to definitively define the mechanism behind a particular phenotype associated with a specific VWF mutation would require in vitro expression studies, which is not feasible given the large number of VWF mutations that continue to be identified.6,7
Perhaps the most striking use for the VWFpp assay is to identify true type 3 VWD patients. Sanders and coworkers assessed VWFpp levels in type 3 VWD patients (VWF:Ag <5 IU/dL).1 Although the majority of patients had undetectable VWFpp levels, VWFpp was detectable in 41% of patients, indicating that VWD in these patients was a result of extremely rapid clearance of VWF (type 1C VWD). Type 3 VWD patients lack VWF in plasma and also lack a regulated VWF storage pool (Weibel-Palade bodies). In contrast, type 1C VWD patients are expected to have normal regulated storage of VWF. The true type 3 VWD patients in this study were found to have a more severe bleeding phenotype than the VWD patients with increased VWF clearance, which may reflect the absence of a VWF storage pool.
The assay of VWFpp is clinically significant because identification of patients with reduced VWF survival affects treatment of these patients. Desmopressin is the most common treatment of type 1 VWD, releasing VWF from Weibel-Palade bodies to increase plasma VWF levels.8 Although type 1C VWD patients may release stored VWF, the released VWF is rapidly cleared from circulation. The VWF released in these patients may be sufficient to achieve hemostasis in minor bleeding situations but may not be adequate for surgery or major bleeding. Type 3 VWD patients require VWF replacement therapy rather than desmopressin administration. The routine assay of VWFpp in patients with VWF levels consistent with type 3 VWD (<5 IU/dL) is likely to lead to reclassification of many as having type 1C VWD, which may expand the treatment options.
In conclusion, data by Sanders and coworkers1 highlight the utility of the assay of VWFpp level, VWFpp/VWF:Ag ratio, and FVIII:C/VWF:Ag ratio in suspected VWD patients. The assay of VWFpp is not routinely included in the diagnostic workup for VWD; however, the data presented by Sanders and coworkers, together with other previous publications, suggest this assay would be a valuable addition to the diagnostic panel, particularly in patients with low VWF:Ag levels. Although there may be limited utility in the diagnosis of type 2 VWD, the ability to identify true type 3, type 1C, and other type 1 VWD patients is clinically important with implications for therapeutic treatment.
Conflict-of-interest disclosure: The author declares no competing financial interests.