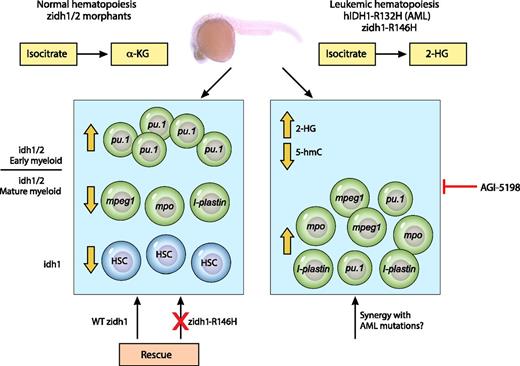
In this issue of Blood, Shi et al describe the role of isocitrate dehydrogenase 1 (idh1) and idh2 in developmental hematopoiesis and demonstrate the conserved leukemogenic potential of human IDH1 mutations in zebrafish.1
Disruption of the function of zidh1/2 enzymes in zebrafish results in partial block of myeloid differentiation, whereas zidh1 also controls the emergence of definitive hematopoietic stem cells. Disease-associated mutations can recapitulate the mammalian phenotype but cannot rescue the mutant phenotypes. α-KG, α-ketoglutarate; 2-HG; 2-hydroxyglutarate; 5-hmC, 5-hydroxymethylcytosine; HSC, hematopoietic stem cell; WT, wild-type. Professional illustration by Patrick Lane, ScEYEnce Studios.
Disruption of the function of zidh1/2 enzymes in zebrafish results in partial block of myeloid differentiation, whereas zidh1 also controls the emergence of definitive hematopoietic stem cells. Disease-associated mutations can recapitulate the mammalian phenotype but cannot rescue the mutant phenotypes. α-KG, α-ketoglutarate; 2-HG; 2-hydroxyglutarate; 5-hmC, 5-hydroxymethylcytosine; HSC, hematopoietic stem cell; WT, wild-type. Professional illustration by Patrick Lane, ScEYEnce Studios.
Understanding the physiological roles of disease-implicated molecules is crucial for dissecting their function and identifying new therapeutic avenues that can lead to a cure. A number of studies have implicated the IDH enzymes in the pathogenesis of acute myeloid leukemia (AML).2 How these enzymes function in normal hematopoiesis during development, if at all, is currently unknown. Shi et al use the zebrafish as an easy genetic model to address this question.
Analysis of expression patterns and genomic locations of zebrafish (z)idh1/2 shows conservation of these enzymes between zebrafish and mammalian IDH1/2. Using genetic approaches, the authors demonstrated that morpholino-injected embryos knocked down for zidh1/2 and the genetic mutant of zidh1 disrupt primitive myelopoiesis. Although the myeloid marker pu.1 was upregulated, myeloid markers of more differentiated cells were decreased, providing a hint for the involvement of zidh1/2 in myeloid differentiation (see figure). Interestingly, restoring the expression of zidh1 could reverse this phenotype, but the mutated zidh1 that mimics mutations found in AML patients could not rescue the phenotype. Additionally, zidh1, but not zidh2, modulated the emergence of definitive hematopoietic stem cells, although the molecular mechanisms underlying this phenomenon remain unknown. Together, these data clearly implicate IDH enzymes in hematopoietic formation and differentiation during development in zebrafish.
Because these enzymes are involved in AML, recreating the disease in zebrafish can provide useful insight into leukemic conservation between species. To this end, we know from other studies that mutations in IDH1 and IDH2 are present in approximately 15% of cytogenetically normal AML samples. In almost all cases, IDH mutations are heterozygous, result in a gain-of-function phenotype, and occur mostly in 3 conserved arginine residues: IDH1-R132, IDH2-R140, and IDH2-R172.3 Normally, these enzymes catalyze the oxidative decarboxylation of isocitrate and produce α-KG in a manner dependent on reduced NAD phosphate.4,5 The mutations observed in AML patients confer a neomorphic enzymatic activity that leads to the reduction of α-KG to 2-HG. 2-HG competes with α-KG as cofactor for various enzymes, including ten-eleven translocases and JmjC histone demethylases, and abrogates their function. This results in epigenetic deregulation that contributes to AML pathogenesis, at least in part through impaired hematopoietic differentiation.6 The impact of IDH mutations in patients’ survival is not really clear, but it has been proposed that 2-HG levels can be used for screening and prognostic purposes.
Many questions clearly arise from the evidence above. Can IDH mutations confer to a leukemogenic phenotype in zebrafish? Is there conservation of leukemogenic potential between species? The authors readily answer these questions by providing evidence that expression of a mutant human IDH1 or its zebrafish orthologue recapitulates the phenotypic characteristics of human AML patients, such as increased levels of 2-HG, reduced 5-hydroxymethylcytosine, and expansion of progenitor hematopoietic cells. IDH-specific inhibitors like AGI-5198 could reverse the phenotype caused by the human mutation, indicating that drugs can be easily tested in a zebrafish model (see figure). Similar results were obtained with a mouse IDH1-R132H hematopoietic-specific knockin. Although this mutant displayed all the phenotypic features of AML patients, it failed to develop leukemia.7
Even though this study is a clear step toward our understanding of the role of IDH enzymes in normal and malignant hematopoiesis, new challenges emerge. The authors provide ample evidence for the role of IDH enzymes in developmental hematopoiesis, but it would be interesting to study adult zidh knockout and mutant knockin animals. What kind of developmental pathways are perturbed in zidh knockouts? Recently, IDH enzymes have been implicated in deregulation of mitochondrial function involving BCL-2 as a potential therapeutic target.8 Is this phenotype recapitulated in zebrafish? The answers to these questions will shed additional light on the physiological roles of IDH enzymes and provide added evidence for the validity of modeling human diseases in zebrafish. Such evidence will open avenues to use the large-scale in vivo chemical screening capabilities of zebrafish for discovering novel therapeutic strategies. In addition, the ease of genetic manipulation of the zebrafish makes it an ideal model to study the cooperative effects of IDH enzymes with other genetic aberrations in a large-scale manner.9 This model can be used to perform studies on the role of IDH mutations in AML initiation, maintenance, and progression, as was done for IDH2-R140Q in mice.10 In summary, the work from Shi et al offers the basis for a genetic model that can shed light on various aspects of AML.
Conflict-of-interest disclosure: The author declares no competing financial interests.