In this issue of Blood, Mallampati et al report on the discovery of a new mechanism of tyrosine kinase inhibitor (TKI) resistance, which is mediated through TKI-mediated priming of mesenchymal stem cells (MSCs) in the bone marrow (BM).1
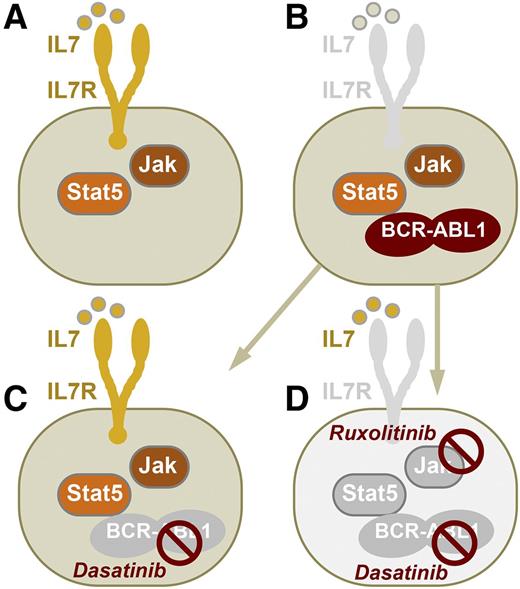
Survival and proliferation of normal mouse pre-B cells are promoted by IL-7R signaling and activation of JAK and STAT5 (A). After malignant transformation of pre-B cells by BCR-ABL1 (Ph+ ALL), IL-7R signaling becomes redundant in ALL cells (B). Mallampati et al show that TKIs of BCR-ABL1 (eg, dasatinib) may effectively block BCR-ABL1 tyrosine kinase activity but concurrently induce IL-7 secretion in MSCs. TKI-induced IL-7 secretion by MSCs can, hence, restore IL-7R–mediated survival and proliferation signaling in Ph+ ALL cells (C). Although reinstatement of IL-7R and JAK signaling as in normal pre-B cells induces drug resistance, dual targeting of both BCR-ABL1 (eg, dasatinib) and JAK (eg, ruxolitinib) activity can prevent IL-7R-JAK–dependent survival signaling and short-circuit drug resistance (D).
Survival and proliferation of normal mouse pre-B cells are promoted by IL-7R signaling and activation of JAK and STAT5 (A). After malignant transformation of pre-B cells by BCR-ABL1 (Ph+ ALL), IL-7R signaling becomes redundant in ALL cells (B). Mallampati et al show that TKIs of BCR-ABL1 (eg, dasatinib) may effectively block BCR-ABL1 tyrosine kinase activity but concurrently induce IL-7 secretion in MSCs. TKI-induced IL-7 secretion by MSCs can, hence, restore IL-7R–mediated survival and proliferation signaling in Ph+ ALL cells (C). Although reinstatement of IL-7R and JAK signaling as in normal pre-B cells induces drug resistance, dual targeting of both BCR-ABL1 (eg, dasatinib) and JAK (eg, ruxolitinib) activity can prevent IL-7R-JAK–dependent survival signaling and short-circuit drug resistance (D).
B-cell lineage acute lymphoblastic leukemia (ALL) cells carrying the Philadelphia chromosome (Ph+) are addicted to BCR-ABL1. Thereby, the oncogenic BCR-ABL1 kinase mimics constitutively active cytokine signaling, which promotes proliferation and survival in normal B-cell precursors. Ph+ ALL almost invariably acquires resistance to BCR-ABL1 TKIs. The mechanisms of TKI resistance are not fully understood.
MSCs in the BM niche have been implicated as enablers of transformation and drug resistance of leukemia cells in multiple ways. For instance, MSCs were identified as the main source of asparagine,2 which promote resistance against l-asparaginase in ALL treatment. Specifically in Ph+ ALL, CXCR4-CXCL12 interactions between leukemia and BM stroma cells, derived from MSCs, were identified as a major factor of TKI resistance.3 Likewise, N-cadherin and β-catenin signaling in MSCs strongly protect leukemia cells against TKIs.4 In addition, defective innervation of MSCs by sympathetic nerve fibers represents a key regulator of leukemic transformation.5 These and other defects in leukemia-associated MSCs can be transmitted to normal MSCs and contribute to a leukemia-specific niche.6 In some cases, MSCs are carriers of driver oncogenes in human ALL, eg, MLL-AF4,7 and oncogenic activation of β-catenin in MSC-derived osteoblasts alone is sufficient to drive leukemogenesis.8 Together, these observations strongly support the concept that MSC and MSC-derived cells in the BM niche are critical mediators of leukemic transformation and drug resistance in both myeloid and B-cell lineage malignancies.
Mallampati et al1 directly measured responses of MSCs, rather than responses of ALL cells, to TKI treatment. Although this approach seems counterintuitive given that ALL cells (not MSCs) are the targets of drug treatment, it represents the logical consequence of a scenario in which MSCs play a central role in leukemia drug resistance. Indeed, their investigation led to the novel and unexpected finding that TKI treatment primed MSCs to secrete cytokines, including interleukin (IL)-7, and to express adhesion molecules that promote survival of ALL cells. Coculture experiments demonstrated that TKI-primed MSCs could specifically enable ALL cells to switch from BCR-ABL1- to IL-7/Janus kinase (JAK)-dependent survival signaling.
Although TKI treatment extinguishes oncogenic BCR-ABL1 kinase activity in Ph+ ALL cells, its effects on MSCs restore normal cytokine signaling as in normal pre-B cells (see figure). Mallampati et al made the striking observation that TKI-primed MSCs enable Ph+ ALL cells to survive biochemical ablation of oncogenic BCR-ABL1 activity. And although TKI treatment removes BCR-ABL1 kinase activity, it primes MSCs to allow Ph+ ALL to revert to a nontransformed state as in normal pre-B cells, which depends on cytokine signaling (eg, IL-7) and JAK activation.1 Previously identified mechanisms of TKI-resistance in Ph+ ALL involve BCR-ABL1 mutations that prevent biochemical kinase inhibition and TKI-mediated survival signaling through activation of B-cell lymphoma 6.9 The study by Mallampati et al demonstrates an unexpected side-effect of TKI treatment on MSCs that enables TKI resistance through the reinstatement of normal pre–B-cell cytokine signaling in Ph+ ALL cells. In genetic experiments based on inducible ablation of IL-7 signaling in Il7fl/fl BCR-ABL1–transformed ALL cells, the authors demonstrate the critical importance of this pathway. Indeed, IL-7–IL-7R signaling is a critical mediator of survival signaling in mouse pre-B cells. However, this is not the case in human B-cell development, and the MSC-secreted cytokine(s) that enable TKI resistance in human Ph+ ALL remain to be identified. Like IL-7 in mouse pre-B ALL cells, MSC-derived cytokines that enable TKI resistance in human Ph+ ALL likely signal through JAK activation. Importantly, the authors demonstrate that inhibition of JAK activity in combination with BCR-ABL1 inhibition can effectively short-circuit MSC-mediated TKI resistance (see figure). This concept is timely and important because it provides a mechanistic rationale for recent preclinical studies10 suggesting that combinations of dasatinib (BCR-ABL1-TKI) and ruxolitinib (a JAK inhibitor) may be effective in preventing drug resistance in Ph+ ALL.
Conflict-of-interest disclosure: The author declares no competing financial interests.