Key Points
In the 3 months after isolated SVT, the risk of a deep venous event or pulmonary embolism is 3.4%.
This risk remains fivefold increased more than 5 years after the superficial event.
Abstract
Recently, it has become apparent that superficial vein thrombosis (SVT) can have serious complications. However, the magnitude of the risk of subsequent deep venous and arterial thrombotic events remains unknown. We examined this in a nationwide population-based setting during a period when SVT was not treated routinely with anticoagulants. The Danish National Registry of Patients, covering all Danish hospitals, was used to identify 10 973 patients with a first-time diagnosis of SVT between 1980 and 2012. A comparison cohort of 515 067 subjects, matched by age, gender, and calendar year, was selected from the general Danish population. Outcomes were venous thromboembolism, acute myocardial infarction, ischemic stroke, and death. During median follow-up of 7 years, the incidence rate of venous thromboembolism was 18.0/1000 person-years (95% confidence interval [CI], 17.2-18.9). The highest risk occurred in the first 3 months (3.4%; 95% CI, 3.0-3.7). Compared with the general population, the hazard ratio was 71.4 (95% CI, 60.2-84.7) in this period, steadily decreasing to 5.1 (95% CI 4.6-5.5), 5 years after the SVT. The hazard ratios for acute myocardial infarction, stroke, and death were 1.2 (95% CI, 1.1-1.3), 1.3 (95% CI, 1.2-1.4), and 1.3 (95% CI, 1.2-1.3), respectively, with the highest risk also shortly after SVT. These data indicate the prognostic importance of SVT and may form the basis for clinical decision-making regarding anticoagulation.
Introduction
Superficial vein thrombosis (SVT) is a relatively common condition of which the incidence was recently established to be about 0.6 per 1000 person-years (py).1 In the past, SVT has been considered a benign, self-limiting disorder, requiring only symptomatic treatment.2,3 However, recent evidence showing that SVT is closely linked to occurrence of deep vein thrombosis (DVT) or pulmonary embolism (PE) indicates the relevance of this condition.4 Three possible types of associations between SVT and DVT/PE can be distinguished.5 First, DVT or PE can be present concomitantly with the superficial event; this has been found in up to 29% of patients presenting with acute SVT.4,6 Second, DVT or PE can develop shortly after a patient initially presents with an isolated superficial event. In a study of 600 patients with symptomatic SVT, but no other thromboembolic events, 18 patients (3%) developed DVT or PE within 3 months, despite most having received anticoagulants.4 Third, patients with a history of SVT may have a four- to sixfold increased lifetime risk of DVT or PE.7,8
This close association between SVT and DVT/PE prompted the Comparison of Arixtra in Lower Limb Superficial Vein Thrombosis with Placebo (CALISTO) trial, which showed that a 45-day anticoagulant treatment regimen after SVT is effective and safe in preventing serious thrombotic events in the 3 months after diagnosis.9 Two other trials have confirmed these findings.10,11 As a result, current guidelines now recommend this treatment regimen for patients with SVT of at least 5 cm in length on a lower limb.12
Recently, Prandoni and colleagues performed a subanalysis of CALISTO trial data to examine whether SVT is associated with increased risk of subsequent arterial cardiovascular events.13 The impetus for their research arose from the recently described association between venous and arterial thrombotic events.14 However, such a relation could not be demonstrated, with a relative risk of 0.97 for arterial cardiovascular events in SVT patients compared with controls.13
Although the studies described here offer some insight into the risk of DVT, PE, or arterial events after a SVT diagnosis, they have limitations, such as inclusion of patients with concurrent DVT or small or selected populations (trial or specialist referral settings). We therefore set out to study the association in a large, unselected population (ie, the entire population of Denmark). The size of the study population allowed accurate estimation of absolute and relative risks as well as several subgroup analyses. Our study focused on patients with a first-time diagnosis of SVT without concurrent DVT or PE. We examined risks of subsequent DVT, PE, acute myocardial infarction (AMI), stroke, and death over different periods.
Methods
Setting
We obtained data from the Danish National Registry of Patients, which has recorded virtually all acute care hospital discharges since 1977 and visits to outpatient specialist clinics and emergency rooms since 1995.15 Our source population consisted of the entire cumulative population of Denmark between 1980 and 2012 (7.1 million inhabitants). In all Danish medical registries, patients are identified through their civil registration number. These unique identifiers are assigned at birth and stored in the Danish Civil Registration System (DCRS) along with date of birth, residency status, and dates of immigration, emigration, and death. Their use allows unambiguous linkage among registries. The data in the DCRS are virtually complete and highly accurate.16
Study population
Cohort of patients with SVT.
We identified all individuals with a first recorded diagnosis of SVT (inpatients and patients treated in hospital outpatient clinics) between 1980 and 2012. We used International Classification of Diseases, 8th revision (ICD-8), codes until December 31, 1993, and ICD-10 codes thereafter (see the ICD Appendix for codes). Patients diagnosed during an emergency room visit were excluded from the cohort because of the low positive predictive value (PPV) of an SVT diagnosis in this setting.17 We also excluded patients who were diagnosed with a DVT within 1 week of their SVT diagnosis date to avoid misclassification of SVTs that were actually DVTs.
Population comparison cohort.
A comparison cohort was sampled from the DCRS. For each patient in the SVT cohort, 50 SVT-free general population cohort members were selected from persons alive on the date of the SVT diagnosis (index date) and matched by age and gender. Follow-up of persons in the comparison cohort was terminated if they developed SVT, in which case they started contributing person-time to the SVT cohort.
Sensitivity analyses.
To further maximize the likelihood of restricting the cohort to patients with isolated SVT, we performed a sensitivity analysis focusing on the period between 2004-2012 for which information on anticoagulation therapy was available from the Danish National Database of Reimbursed Prescriptions.18 Because patients with SVT were generally not treated with anticoagulant therapy during this time, we aimed to exclude possibly misclassified or concomitant DVT by excluding patients from both cohorts who had a redeemed prescription for an anticoagulant within 1 month before or 1 week after their SVT diagnosis date or the index date. In another sensitivity analysis focusing on the period between 2002-2012, we included only patients with a deep vein ultrasound scan within 1 week before or after their SVT diagnosis date or the index date in the comparison cohort.
Exclusions.
Subjects from the SVT and general population comparison cohorts who had a prior diagnosis of venous thromboembolism (VTE), AMI, or stroke (on or before the SVT diagnosis date) were excluded.
Study outcomes
Members of the 2 cohorts were linked to the DCRS and the Danish National Registry of Patients to identify all inpatient and outpatient diagnoses of DVT, PE, AMI, and ischemic stroke as well as death (see the ICD Appendix for codes). All diagnoses from emergency room visits were excluded because of their low PPV.17 Both primary and secondary diagnoses were included.
Confounders
Clinical variables that were related to occurrence of SVT and that were risk factors for any of the study outcomes were considered possible confounding factors. These variables included cancer, pregnancy, fracture, surgery, Charlson Comorbidity Index (CCI) score, and autoimmune disease (see the ICD Appendix for a list of diagnoses). Transient confounders, such as pregnancy, fracture, or surgery, were only considered as such when they occurred around the time of the SVT. When such an event arose later during follow-up and shortly before an outcome arose, it was used to classify the outcome (provoked vs unprovoked event) and not treated as a possible confounder.
Statistical analysis
We followed both cohorts from SVT diagnosis/index date until emigration, death, end of follow-up (December 31, 2012), or the occurrence of a study outcome, whichever occurred first. We calculated the rate of DVT, PE, AMI, stroke, and mortality for the SVT patients and members of the population comparison cohort. Rates were expressed as number of events per 1000 py. We used Kaplan-Meier analysis to construct survival curves, treating death as competing risk, and to estimate risks of each outcome. We used Cox regression to compute the hazard ratio (HR) with accompanying 95% confidence intervals (CI) as measures of relative risk for the endpoint analyses. The VTE outcome was disaggregated into provoked and unprovoked VTE and into DVT and PE. Provoked VTE was defined as all venous thrombotic events occurring within 3 months after surgery, pregnancy, or fracture or when a cancer diagnosis was present in the period of 1 year before and 3 months after the VTE diagnosis date. All other venous thrombotic events were considered unprovoked. All analyses were adjusted for age, gender, and calendar time by study design. We also adjusted for the possible confounders described previously. We stratified by time between index date and study outcome date as follows: 3 months, >3 months to 1 year, >1 year to 5 years, and more than 5 years. Finally, we conducted subgroup analyses for men and women separately as well as for patients without a cancer diagnosis 1 year before and 1 year after the SVT/index date. Analyses were performed using SAS 9.2 (SAS Institute Inc., Cary, NC). The study was approved by the Danish Data Protection Board (record number 1-16-02-1-08) and was conducted in accordance with the Declaration of Helsinki.
Results
Patient characteristics
We identified 10 973 subjects with a diagnosis of SVT, whereas the population comparison cohort (matched by gender, age, and index date) consisted of 515 067 subjects. The proportion of women was ∼60% in both cohorts. The median age was 61.7 years (interquartile range 47.5-73.3 years) in the SVT cohort and nearly the same in the general population cohort. In the SVT cohort, 20 patients emigrated during the study period (0.18%); in the comparison cohort, 2505 subjects (0.49%) emigrated during this period. Compared with the population cohort, SVT patients had more cancer diagnoses in the period 1 year before to 1 year after the index date (9.2% vs 2.8%), had been pregnant more often within 3 months before the index date (4.5% vs 0.6% of all women), had more recent fractures (5.6% vs 1.6%) and surgery (19.2% vs 3.9%), had more comorbidity (CCI score >2: 7% vs 3.7%) and were more likely to have autoimmune disease (8.2% vs 4.9%). Patient characteristics are shown in Table 1.
DVT
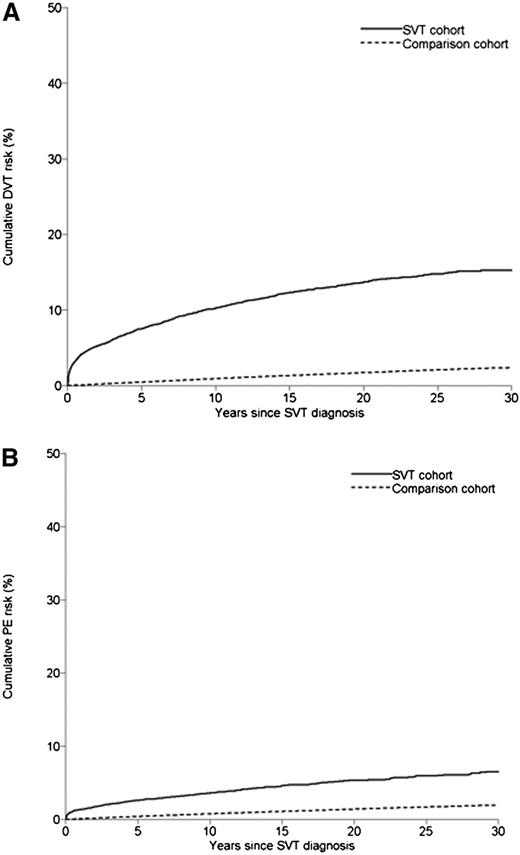
Of the patients with SVT, 1170 developed DVT during a median follow-up of 6.4 years, leading to an incidence rate of 12.8 per 1000 py (95% CI 12.1-13.6). In the comparison cohort, 6096 developed DVT during a median follow-up of 8.4 years, with a corresponding incidence rate of 1.2 per 1000 py (95% CI 1.1-1.2). This yielded an age- and gender-adjusted HR of 11.8 (95% CI 11.1-12.6), with little change after adjustment for cancer, pregnancy, fracture, surgery, CCI score, and autoimmune disease (HR 11.3; 95% CI 10.5-12.1) (Table 2). Table 3 provides cumulative incidences; Table 4 shows HRs stratified by follow-up time. The risk of DVT after SVT was highest during the first 3 months of follow-up (incidence: 2.5%, 95% CI 2.2-2.8; adjusted HR: 87.7, 95% CI 70.8-108.6), decreasing steadily to a still considerably increased risk after 5 years of follow-up (adjusted HR: 6.3, 95% CI 5.6-7.0) (Figure 1A).
Cumulative incidence of DVT and PE in patients with SVT and in members of the general population comparison cohort. (A) DVT. (B) PE.
Cumulative incidence of DVT and PE in patients with SVT and in members of the general population comparison cohort. (A) DVT. (B) PE.
Two sensitivity analyses were performed to prevent misclassification of DVT as SVT. The first sensitivity analysis, excluding patients who had been using anticoagulants between 1 month before and 1 week after the date of SVT diagnosis or the index date, yielded a slightly higher adjusted risk estimate overall (HR: 15.8, 95% CI 13.6-18.5) as well as for the separate periods: HR for the first 3 months: 97.7 (95% CI 65.6-145.4), declining steadily to an HR for >5 years of 6.6 (95% CI 4.1-10.7) (see supplemental Table 1 on the Blood Web site). The second sensitivity analysis, restricted to patients who had an ultrasound scan within 1 week before or after their SVT admission date or the index date, also showed higher risk estimates, with an overall adjusted HR of 17.8 (95% CI 15.6-20.3) and HRs for the first and last periods of 111.5 (95% CI 78.5-158.5) and 7.7 (95% CI 5.6-10.7), respectively (supplemental Table 2).
To study the effect of cancer on our risk estimates, we excluded all patients diagnosed with any form of cancer within 1 year before or 1 year after the SVT or index date. This did not change the effect estimate, with the HR remaining at 11.4 (95% CI 10.6-12.3), again with highest risk occurring in the period shortly after the SVT (supplemental Table 3). In a subgroup analysis stratified by gender, we found that the association between SVT and subsequent DVT was stronger in men than in women (overall HR for men: 14.3 [95% CI 13.0-15.9] vs 9.3 [95% CI 8.4-10.2] for women) (Table 5).
PE
The incidence rate of PE was clearly lower than that of DVT. In patients with SVT, the incidence rate of PE was 4.5 per 1000 py (95% CI 4.1-4.9) compared with 0.9 (95% CI 0.9-1.0) in the general population cohort (Table 2). This yielded an age- and gender-adjusted HR of 4.9 (95% CI 4.4-5.4), which was affected little by adjustment for possible confounders (HR 4.5; 95% CI 4.1-5.0) (Table 2). As in the case of DVT, the risk was highest during the first 3 months of follow-up (0.9% [95% CI 0.7-1.1]; adjusted HR: 45.4 [95% CI 33.9-60.9]). This risk steadily decreased to an HR of 2.9 (95% CI 2.5-3.5) after 5 years of follow-up (Figure 1B). The relationship was stronger in the sensitivity analyses excluding patients who received anticoagulation therapy (overall HR: 6.4; 95% CI 5.1-8.0) and restricted to patients who had received an ultrasound scan (overall HR: 5.5; 95% CI 4.5-6.7) (supplemental Tables 1 and 2). Excluding patients with cancer had little effect (supplemental Table 3). Finally, as for DVT, the association between SVT and subsequent PE was stronger in men than in women (HR: 5.8 [95% CI 5.0-6.8] in men overall vs 3.8 [95% CI 3.3-4.4] in women overall) (Table 5).
Provoked vs unprovoked VTE
We examined differences between provoked and unprovoked venous thrombotic events, aggregating DVT and PE as VTE. The risk was 2.0% for unprovoked events vs 1.3% for provoked events in the first 3 months following SVT, with adjusted HRs of 69.9 and 59.2 in this follow-up period, respectively (Tables 3 and 4). Adjustment for possible confounders substantially attenuated the HRs only for provoked events (Tables 2 and 4, with crude HRs for the periods for provoked VTE of: 95.4 for 0 to 3 months, 17.8 for 3 months to 1 year, 5.5 for 1 to 5 years, and 3.6 for >5 years; crude HRs for the other outcomes not shown because they barely differed from the adjusted).
AMI and stroke
Over a median follow-up of 7.0 years, 562 patients in the SVT cohort developed an AMI, yielding an incidence rate of 5.8 per 1000 py (95% CI: 5.3-6.3). The corresponding rate in the general population cohort was 4.8 (95% CI: 4.8-4.9). This led to an age- and gender-adjusted HR of 1.2 (95% CI 1.1-1.3), which did not change after adjustment for possible confounders. For ischemic stroke, the rates were slightly higher in both cohorts, but the HRs were similar to those for AMI. The incidence rate was 7.2 (95% CI: 6.7-7.8) per 1000 py in the SVT group and 5.5 (95% CI: 5.5-5.6) in the general population cohort, yielding an unadjusted HR of 1.4 (95% CI 1.3-1.5) and an adjusted HR of 1.3 (95% CI 1.2-1.4) (Table 2). For both AMI and ischemic stroke, the HR was highest in the first 3 months after the SVT diagnosis (1.6 [95% CI 1.0-2.5] for AMI and 2.6 [95% CI 1.8-3.8] for ischemic stroke, decreasing over time to 1.2 [95% CI 1.0-1.3] for AMI and 1.3 [95% CI 1.1-1.4] for ischemic stroke 5 or more years after the SVT). The HRs were similar in the sensitivity analyses (supplemental Tables 1 and 2) and in the analysis excluding cancer patients (supplemental Table 3). A slightly higher risk was observed in men than in women (Table 5).
Mortality
During a median follow-up period of 7.2 years, 4475 SVT patients died, yielding a mortality rate of 45.1 (95% CI: 43.8-46.4) per 1000 py. During a median follow-up period of 8.4 years for the general population cohort, the mortality rate was slightly lower, at 33.4 (95% CI: 33.3-33.6). The unadjusted HR was 1.4 (95% CI 1.4-1.5) and the adjusted HR was 1.3 (95% CI 1.2-1.3). We found a gradient in risk over time, with an adjusted HR of 3.5 (95% CI 3.1-4.0) in the first 3 months after SVT and of 2.2 (95% CI 2.0-2.4) in the 3-month to 1-year period. Subsequently, risks were only minimally increased (Tables 3 and 4). The HR was somewhat lower after excluding cancer patients (supplemental Table 3), but were essentially unchanged in the 2 sensitivity analyses (supplemental Tables 1 and 2). We found no clear difference in mortality between men and women (Table 5).
Discussion
In this nationwide population-based study, we found a risk of 2.5% for subsequent DVT and of 0.9% for PE in patients who presented with an isolated SVT in the first 3 months after the SVT. The risk of subsequent AMI or ischemic stroke was slightly higher in SVT patients, as was their risk of death. The relation between SVT and risk of VTE was time-dependent, with a 70-fold increased risk of VTE in the first 3 months after SVT, declining gradually to a long-term fivefold increased risk after 5 years. These results remained robust in several sensitivity analyses. All HRs for VTE were about 1.5-fold higher in men than in women.
In recent years, data have accumulated on the seriousness of SVT. Having been considered a benign and self-limiting disease, not normally needing treatment, several large studies have shown that the risk of concomitant or subsequent DVT or PE is substantial, and that anticoagulant treatment is beneficial in preventing progression to a more serious thromboembolic event.4,6,9 In our study, we focused on isolated SVT, excluding concomitant DVT or PE, and examined immediate and longer-term VTE risk during a period when SVT was not treated with anticoagulant therapy. Our findings suggest 2 conclusions about the relation between superficial and deep venous events. First, the immediate risk (within 3 months) of DVT is high (ie, 2.5% and almost 90 times increased). The immediate risk of PE is also elevated, although to a lesser extent (0.9% and 45-fold increased). Apparently, a superficial thrombus easily extends into a clot in the deep veins, which may subsequently embolize. Second, although these risks attenuate over time, they remain 3- to sixfold increased even after 5 years. Our findings are in accord with earlier studies showing VTE risks of 3% to 4% in the first 3 months after SVT4,19 as well as a four- to sixfold increased long-term risk for deep venous events in patients with a history of SVT7,8 and suggests that superficial and deep venous events result from a common hypercoagulable state. This thesis is supported by the finding that a DVT occurs in the contralateral leg in up to 10% of cases. The expected percentage would be 0% under the assumption that a DVT occurs only as an extension of the superficial event.20 Furthermore, risk factors for SVT largely overlap with those for DVT, including high body mass index, immobility, and cancer, which also suggests that SVT and DVT have a similar etiology. Some investigators argue that SVT should not be categorized separately from DVT and PE, but rather be considered as part of the venous thrombotic spectrum.21 Results from recent studies, including ours, support this view.
We found a clearly higher prevalence of several classical risk factors for DVT and PE in the SVT cohort compared with the general population cohort, such as cancer, surgery, pregnancy, and fracture. Adjustment for these factors led to attenuation of the HR in the provoked VTE group. This suggests that part of the risk in patients with provoked VTE is explained by these factors, which appear to affect the occurrence of both SVT and DVT/PE. The still considerable HR remaining after adjustment could result from other mutual risk factors for which we did not adjust. An alternative explanation is a direct relation in which DVT/PE results from SVT. Considering that we adjusted for the most common and strongest risk factors for VTE, and in light of the clinical course of SVT,22 the latter mechanism is likely largely responsible for our findings.
In their study of the risk of arterial events in 737 patients with an isolated SVT not involving the saphenofemoral junction,13 Prandoni and colleagues found no increased risk compared with controls. In our study, we found a slightly higher risk of AMI and stroke subsequent to SVT (HR of 1.2 and 1.3, respectively). As in the case of VTE, these risks were highest in the first 3 months after SVT (HR of 1.6 and 2.6, respectively). However, as described previously, the results of our sensitivity analyses suggest that these relative risks may be somewhat lower, with possibly no effect on AMI. In Prandoni et al’s study, a slightly higher risk was also found for stroke compared with AMI among patients with SVT (1.6% vs 1.3%), so a weak association is more likely between SVT and stroke than between SVT and AMI.
Our study showed that risk of death in SVT patients was increased to a similar extent as for AMI and stroke, again with the highest risk close in time to the SVT event. When we excluded cancer patients, the HR was slightly reduced during the first year after SVT. We lacked information on causes of death, so can only speculate that PE, AMI, and stroke all may have contributed to the slightly increased mortality.
We found that the relative risks of all thromboembolic outcomes were higher in men. This is remarkable considering that about 60% of SVT patients are female.4,9 An increased risk of DVT/PE in men with SVT has been described in the POST study4 and in the Superficial Thrombophlebitis Treated by Enoxaparin Study Group trial.23 The higher risk in men than in women of recurrent VTE has been recognized for some time,24 but only recently has it become clear that this disparity in risk also exists for first events.25 Although the cause of the risk difference between the genders is not yet known, our results are in line with these findings.
While our study sheds light on the pathophysiology and natural course of SVT, it also underscores the clinical importance of anticoagulant treatment to prevent further extension of the superficial thrombus and development of a subsequent DVT or PE. Two recent trials that demonstrated clear beneficial effects of anticoagulant treatment showed that treatment of at least 30 days was necessary.9,10 The long-term increased risk should be considered particularly in patients with a history of SVT when they are exposed to risk factors for VTE, such as use of oral contraception or a need to undergo surgery. Recently, relative risks of 9 to 50 for occurrence of VTE have been described in patients who had previous SVT and were exposed to such acquired risk factors.26
Strengths of our study were its large sample size and its unselected population, leading to precise estimates overall and in the many subgroups examined. Use of computerized registries with nationwide coverage assured virtually complete collection of clinical data.16 Sufficient additional information was available to allow adjustment for several strong confounders as well as 2 sensitivity analyses.
A study limitation is the lack of detailed information on the extent of the superficial thrombus or the site of the affected leg. We therefore could not study a possible temporal relation between size and location of the superficial event and subsequent occurrence of DVT. Another concern is that we may have missed other confounding factors, such as oral contraceptive use, in the relation between superficial and deep venous events. However, because the relation remained strong after we adjusted for the primary VTE risk factors, it is unlikely that it can be fully explained by confounding. Another possible limitation is exclusion of SVT diagnoses made in the emergency room, which could have led to loss of information. However, emergency room diagnoses generally represent temporary “working diagnoses,” which are not updated with final diagnoses (often determined much later). Because the inaccuracy of emergency room diagnoses would have led to misclassification and dilution of the true risks, we decided to exclude them. Furthermore, because our study was based on registry data, we cannot exclude misclassification of SVT or the outcome diagnoses. Although the latter diagnoses have been validated several times, with a PPV of about 75%,17,27 SVT has been validated once in a study of pregnant and postpartum women.28 In that study, a high PPV of 89.6% (95% CI 84.3-95.0) was found for SVT. The study’s reported PPVs for DVT (74.5 [95% CI 66.8-81.2]) and PE (63.6 [95% CI 40.7-82.8]) did not differ greatly from other validation studies not restricted to pregnant/postpartum women (DVT: 71.3 [95% CI 67.4-75.0]; PE: 82.1 [95% CI 77.2-86.4]).17 This suggests that the PPV for SVT was also quite accurate. We took several further precautions in our study to ensure that events classified as SVTs were not in fact DVTs. We excluded all DVTs occurring within a week of an SVT and performed 2 sensitivity analyses, which yielded slightly higher HRs than in the main analysis. If some SVTs in fact had been DVTs, we would have overestimated the risk of DVT, and it would be expected that in the more strictly construed sensitivity analyses, the HRs would decrease. Because the opposite occurred, we are quite confident that misclassification of DVTs as SVTs was minimal. Finally, we excluded patients with a history of VTE, AMI, or stroke, but because this history was only available from the start of the study, we may have included a few recurrences (rather than first events only) during the first years of cohort formation.
In conclusion, we found a strong relation between the presence of a superficial thrombosis and subsequent occurrence of a deep venous event in a large, unselected population during a period when SVT was not yet routinely treated with anticoagulants. This relation was strongest in the first months, but remained increased over time. These findings reflect the natural course and the prognostic significance of SVT and emphasize its clinical importance.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The study was supported by the Aarhus University Research Foundation.
Authorship
Contribution: H.T.S. conceived the study idea and developed it in collaboration with the other coauthors; all authors contributed to the design of the study; E.H.-P. and H.T.S. collected the data; S.C.C. and H.T.S. reviewed the literature; all authors directed the analyses,which were carried out by E.H.-P.; all authors participated in the discussion and interpretation of the results; S.C.C. organized the writing and wrote the initial draft; all authors critically revised the manuscript for intellectual content and approved the final version before submission; H.T.S. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Suzanne C. Cannegieter, Department of Clinical Epidemiology, Leiden University Medical Center, PO Box 9600, 2300 RC Leiden, The Netherlands; e-mail: s.c.cannegieter@lumc.nl.