Key Points
Limited-stage diffuse large B-cell lymphoma has good outcomes with CHOP followed by radiotherapy but has a pattern of continuous relapses.
Adding radioimmunotherapy consolidation results in outcomes that are at least as good as with rituximab added to CHOP and radiotherapy.
Abstract
In the S0313 trial, we evaluated the impact of adding ibritumomab tiuxetan consolidation to 3 cycles of standard cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) chemotherapy plus involved field radiotherapy (IFRT) in patients with limited-stage aggressive B-cell non-Hodgkin lymphoma (LD-NHL). Patients with at least 1 stage-modified adverse risk factor (nonbulky stage II, age >60 years, elevated lactate dehydrogenase, or World Health Organization performance status of 2) were treated with CHOP on days 1, 22, and 43, followed 3 weeks later by 40 to 50 Gy of IFRT. An ibritumomab tiuxetan regimen was initiated 3 to 6 weeks following IFRT. Forty-six patients were registered and eligible, with median follow-up of 7.3 years. The progression-free survival estimate is 89% at 2 years, 82% at 5 years, and 75% at 7 years. The overall survival estimate is 91% at 2 years, 87% at 5 years, and 82% at 7 years. Grade 4 adverse events occurring more than once included neutropenia (8), leukopenia (5), and lymphopenia (2). Febrile neutropenia was observed in 4 patients. No cases of treatment-related myeloid neoplasms were noted. In conclusion, patients with high-risk LD-NHL treated with 3 cycles of CHOP plus IFRT followed by ibritumomab tiuxetan consolidation had outcomes that compare favorably to our historical experience. The clinical trial was registered at www.clinicaltrials.gov as #NCT00070018.
Introduction
Patients with aggressive histologies of B-cell non-Hodgkin lymphoma (NHL) having limited-stage disease and without adverse risk factors are generally cured of their disease with brief chemotherapy (3 cycles of cyclophosphamide, doxorubicin, vincristine, and prednisone [CHOP(3)]) followed by involved field radiotherapy (CHOP(3) plus IFRT), with 94% to 97% overall survival (OS) at 10 years.1-3 Patients having adverse clinical risk factors have an excessive relapse rate leading to a 5-year OS of 50% to 77% and a 10-year OS of 0% to 50% depending on the number of adverse risk factors (adverse risk factors include stage II disease, age >60 years, elevated serum lactate dehydrogenase [LDH], and less than full ambulatory performance status).1-5 We have previously shown that the addition of rituximab to CHOP(3) plus IFRT for use in high-risk patients resulted in an improved estimated 4-year progression-free survival (PFS) of 88% and OS of 92%.6 Relapses were largely systemic (5 of 6 evaluable) and continue to be seen with longer follow-up.
Radiotherapy usually sterilizes disease within the treatment volume, and relapses within that area are uncommon in patients with limited disease, if the radiotherapy is given on time and without interruption.6 The ibritumomab tiuxetan regimen (Zevalin) is a radiolabeled anti-CD20 antibody that has shown excellent single-agent activity in rituximab-naive patients with diffuse large B-cell lymphoma (DLBCL).7 This form of radioimmunotherapy (RIT) has the potential to prevent systemic spread and distant relapse of disease. In the SWOG S0313 trial, we tested the effect of adding consolidation with ibritumomab tiuxetan to the “backbone” treatment of CHOP(3) plus IFRT in patients with stage I, or nonbulky stage II, aggressive histologies of B-cell NHL with high-risk features.
Methods
Patient selection
Eligible patients were enrolled using similar selection criteria as in SWOG 8736 wherein CHOP(3) plus IFRT was compared with 8 cycles of CHOP alone (CHOP(8)) and identical criteria to SWOG 0014 wherein rituximab was added to CHOP(3) plus IFRT. Importantly, all patients had an incisional/excisional biopsy diagnosis of aggressive NHL, including diffuse large B-cell, Burkitt, or Burkitt-like, and mantle cell histologies. Diagnoses based on fine-needle aspirations and small-core biopsies were not allowed. No amount of a follicular component was allowed. All pathologic diagnoses were confirmed upon central review. Germinal center B-cell–like (GCB) vs non-GCB designation was based on immunohistochemical stains being positive for CD10 and/or Bcl-6 (GCB) or negative for both CD10 and Bcl-6 (non-GCB).
Patients were eligible if they had stage I/IE disease, or II/IIE disease if the largest diameter of the largest mass measured <10 cm. The extent of disease was determined by physical examination, computed tomography (CT) scans of the chest, abdomen, and pelvis, and a bone marrow biopsy. Positron emission tomography (PET)/CT scans were not required. In addition, patients must have had at least 1 adverse risk factor defined by the stage-modified International Prognostic Index including stage II disease, age >60 years, elevated serum LDH, or a World Health Organization performance score of 2 or higher. The study was approved by the institutional review boards of each participating SWOG institution. All patients gave written informed consent in accordance with institutional guidelines and the Declaration of Helsinki. All authors had access to primary clinical trial data.
Treatment
CHOP chemotherapy was given at standard doses (cyclophosphamide 750 mg/m2 IV, doxorubicin 50 mg/m2 IV, vincristine 1.4 mg/m2 IV [not to exceed 2 mg]), and prednisone 100 mg orally) for 5 consecutive days. Cycles of treatment were given on days 1, 22, and 43. IFRT began 3 weeks after completion of chemotherapy, and consisted of 40 Gy in daily fractions of 1.8 to 2 Gy for patients with a complete response, with an additional 6 to 10 Gy permissible for patients with a partial response. Radiation was directed to lymph node regions or organs involved by overt disease prior to initiation of chemotherapy (involved field radiation therapy). If a boost dose was given, a field (volume) reduction was permissible. Rituximab plus Yittrium90 ibritumomab tiuxetan consolidation was given 3 to 6 weeks following completion of IFRT. Rituximab, 250 mg/m2, was given on day 1 of consolidation, followed within 4 hours by 5 mCi of Indium111 ibritumomab tiuxetan, followed by whole-body gamma camera images to assess biodistribution of the ibritumomab tiuxetan. Scans were obtained at 2 to 24 hours and at 48 to 72 hours. Following acceptable biodistribution, rituximab was repeated on days 7, 8, or 9 followed within 4 hours by RIT, 0.4 mCi/kg, not to exceed 32 mCi total dose. Follow-up examinations and tests including CT scans were repeated every 6 months for the first 2 years and annually thereafter, with all patients followed until death.
Statistical analysis
The primary objective of this study was to estimate the 2-year PFS probability. The original planned accrual goal was 60 eligible patients; however, given slowing accrual, limited improvements to precision of estimates with further enrollment, and no ineligible patients, the study was closed with 46 enrolled patients. We assumed a 2-year PFS of at least 75% based on prior results without rituximab/RIT consolidation; therefore, with 46 patients, a 2-year PFS of ≥84% in this trial would merit further investigation. Forty-six patients allowed us to determine the 2-year PFS estimate to within ±12% (based on an assumed null probability of 75%) using a 1-arm survival design, and to determine the probability of any toxicity to within ±15%. Any adverse event with at least a 5% probability was likely to be seen at least once (91% probability).
The analysis was performed using all eligible patients. Estimates of PFS and OS were performed using the Kaplan-Meier method.8 PFS was measured from the time of registration until disease progression or relapse, or death resulting from any cause. OS was measured from the time of registration until death from any cause. Toxic effects were coded according to the National Cancer Institute Common Toxicity Criteria, version 2.
To better assess the value of adding RIT consolidation, we compared outcomes from S0313 patients to outcomes from similarly staged patients from SWOG 8736 treated with CHOP(3) plus IFRT (n = 68) and from SWOG 0014 treated with 3 cycles of rituximab plus CHOP (R-CHOP(3)) plus IFRT (n = 60). The SWOG 8736 patients must have had 1 or more adverse risk factors and must have had similar histology, whereas eligibility criteria from S0014 were the same as the eligibility criteria for this study.
This report represents data through February 4, 2014.
Results
Patient characteristics
Forty-six patients were registered between March 12, 2004 and October 27, 2008. All patients were eligible. All patients have finished treatment, with 42 of the 46 patients (91%) completing all therapy as planned. Treatment was not completed in 4 patients because of adverse events (n = 2), therapy refusal (n = 1), or progression (n = 1). Clinical characteristics are summarized in Table 1. Thirty-five percent of patients had >1 adverse risk factor, with 57% being at least 60 years of age, 52% with stage II disease, 37% with elevated LDH, and only 4% with World Health Organization performance status of 2 or higher. Twenty percent of patients had systemic symptoms.
Patient characteristics
| Clinical variable . | S0313 (n = 46) . | S0014 (n = 60) . | S8736 (n = 68) . | |||
|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | No. . | % . | |
| Age, y | ||||||
| Median | 61* | 69 | 66 | |||
| Range | 23-85 | 26-85 | 35-85 | |||
| ≥60 | 26 | 57 | 44 | 73 | 52 | 76 |
| Male sex | 30 | 65* | 27 | 45 | 40 | 59 |
| White race | 43 | 93 | 57 | 95 | 63 | 93 |
| Hispanic race | 3 | 7 | 1 | 2 | 2 | 3 |
| B symptoms | 9 | 20 | 14 | 23 | 14 | 21 |
| Histology | ||||||
| DLBCL | 44 | 96 | 56 | 93 | 60 | 88 |
| Burkitt-like | 1† | 2 | 3 | 5 | 7 | 10 |
| High-grade B | 0 | 0 | 1 | 2 | 1 | 1 |
| Mantle cell | 1 | 2 | 0 | 0 | 0 | 0 |
| LDH > ULN | 15 | 33 | 13 | 22 | 17 | 25 |
| Stage II | 24 | 52 | 26 | 43 | 32 | 47 |
| Performance status | ||||||
| 0 | 29 | 64 | 42 | 70 | 51 | 75 |
| 1 | 14 | 31 | 17 | 28 | 15 | 22 |
| 2 | 2 | 4 | 1 | 2 | 2 | 3 |
| Stage-modified IPI risk | ||||||
| 1 | 30 | 65 | 42 | 70 | 40 | 59 |
| 2 | 12 | 26 | 12 | 20 | 21 | 31 |
| 3 | 3 | 7 | 6 | 10 | 7 | 10 |
| 4 | 1 | 2 | 0 | 0 | 0 | 0 |
| Clinical variable . | S0313 (n = 46) . | S0014 (n = 60) . | S8736 (n = 68) . | |||
|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | No. . | % . | |
| Age, y | ||||||
| Median | 61* | 69 | 66 | |||
| Range | 23-85 | 26-85 | 35-85 | |||
| ≥60 | 26 | 57 | 44 | 73 | 52 | 76 |
| Male sex | 30 | 65* | 27 | 45 | 40 | 59 |
| White race | 43 | 93 | 57 | 95 | 63 | 93 |
| Hispanic race | 3 | 7 | 1 | 2 | 2 | 3 |
| B symptoms | 9 | 20 | 14 | 23 | 14 | 21 |
| Histology | ||||||
| DLBCL | 44 | 96 | 56 | 93 | 60 | 88 |
| Burkitt-like | 1† | 2 | 3 | 5 | 7 | 10 |
| High-grade B | 0 | 0 | 1 | 2 | 1 | 1 |
| Mantle cell | 1 | 2 | 0 | 0 | 0 | 0 |
| LDH > ULN | 15 | 33 | 13 | 22 | 17 | 25 |
| Stage II | 24 | 52 | 26 | 43 | 32 | 47 |
| Performance status | ||||||
| 0 | 29 | 64 | 42 | 70 | 51 | 75 |
| 1 | 14 | 31 | 17 | 28 | 15 | 22 |
| 2 | 2 | 4 | 1 | 2 | 2 | 3 |
| Stage-modified IPI risk | ||||||
| 1 | 30 | 65 | 42 | 70 | 40 | 59 |
| 2 | 12 | 26 | 12 | 20 | 21 | 31 |
| 3 | 3 | 7 | 6 | 10 | 7 | 10 |
| 4 | 1 | 2 | 0 | 0 | 0 | 0 |
ULN, upper limit of normal.
Statistically significantly different from study S0014, P < .05.
The only case of Burkitt-like lymphoma in S0313 was reviewed and confirmed to be Burkitt lymphoma.
Toxicity
All 46 eligible patients were evaluable for toxicity. Toxicity of treatment was manageable (see Table 2). Fourteen of 46 patients had at worst grade 4 toxicity, mostly hematologic, and 16 had at worst grade 3 toxicity. The most frequent grade 3 or 4 adverse events were hematologic. Febrile neutropenia was observed in 4 patients (1 grade 4 and 3 grade 3), 3 of them during administration of CHOP (timing of the fourth episode was unknown). Grade 4 neutropenia was observed during CHOP (n = 2) and at 3 months after therapy completion (n = 1). Within 1 month of ibritumomab administration, there were reductions in leukocytes (1 grade 4 and 5 grade 3), lymphocytes (1 grade 4 and 3 grade 3), and platelets (3 grade 3). There have been no patients who developed treatment-related myeloid neoplasms.
Most common toxicities
| . | Any grade . | Grades 3 or 4 . | ||
|---|---|---|---|---|
| No. . | % . | No. . | % . | |
| By individual toxicity type | ||||
| Fatigue | 33 | 72 | 2 | 4 |
| Leukocytes | 30 | 65 | 18 | 39 |
| Hemoglobin | 28 | 61 | 1 | 2 |
| Neutropenia | 27 | 59 | 16 | 35 |
| Febrile neutropenia | 4 | 9 | 4 | 9 |
| Platelets | 26 | 57 | 9 | 20 |
| Alopecia | 24 | 52 | 0 | 0 |
| Nausea | 21 | 46 | 1 | 2 |
| Lymphopenia | 19 | 41 | 7 | 15 |
| Constipation | 16 | 35 | 2 | 4 |
| Radiation dermatitis | 16 | 35 | 1 | 2 |
| Neuropathy-sensory | 14 | 30 | 0 | 0 |
| Hyperglycemia | 12 | 26 | 4 | 9 |
| Vomiting | 11 | 24 | 2 | 4 |
| Dry mouth | 10 | 22 | 1 | 2 |
| By toxicity category | ||||
| Hematologic | 38 | 83 | 26 | 57 |
| Gastrointestinal | 38 | 83 | 8 | 17 |
| Flu-like symptoms | 35 | 76 | 4 | 9 |
| Dermatologic | 34 | 74 | 2 | 4 |
| . | Any grade . | Grades 3 or 4 . | ||
|---|---|---|---|---|
| No. . | % . | No. . | % . | |
| By individual toxicity type | ||||
| Fatigue | 33 | 72 | 2 | 4 |
| Leukocytes | 30 | 65 | 18 | 39 |
| Hemoglobin | 28 | 61 | 1 | 2 |
| Neutropenia | 27 | 59 | 16 | 35 |
| Febrile neutropenia | 4 | 9 | 4 | 9 |
| Platelets | 26 | 57 | 9 | 20 |
| Alopecia | 24 | 52 | 0 | 0 |
| Nausea | 21 | 46 | 1 | 2 |
| Lymphopenia | 19 | 41 | 7 | 15 |
| Constipation | 16 | 35 | 2 | 4 |
| Radiation dermatitis | 16 | 35 | 1 | 2 |
| Neuropathy-sensory | 14 | 30 | 0 | 0 |
| Hyperglycemia | 12 | 26 | 4 | 9 |
| Vomiting | 11 | 24 | 2 | 4 |
| Dry mouth | 10 | 22 | 1 | 2 |
| By toxicity category | ||||
| Hematologic | 38 | 83 | 26 | 57 |
| Gastrointestinal | 38 | 83 | 8 | 17 |
| Flu-like symptoms | 35 | 76 | 4 | 9 |
| Dermatologic | 34 | 74 | 2 | 4 |
Outcomes
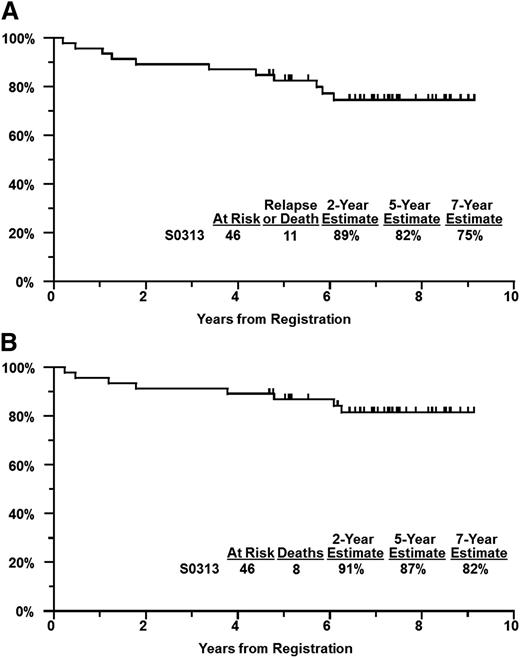
There were no major protocol deviations, defined as incorrect treatment administration or life-threatening dosing errors. Median follow-up among patients still alive is 7.3 years (maximum, 9.1 years). Eleven of the 46 patients have progressed or died. The 2-year PFS estimate was 89% (95% confidence interval [CI], 81%-97%), the 5-year PFS was 82% (95% CI, 71%-94%), and the 7-year PFS estimate was 75% (Figure 1A). Seven patients relapsed, at the median of 3.4 years from registration (range, 0.2-5.9 years). One patient had lymphoma progression the day after starting radiation. Of the remaining 6 relapses, only 1 was in the radiation field whereas the rest were outside of the radiation field. All 6 of these patients had lymphoma presenting in the head and neck region. Eight patients have died, including 5 from cancer (lymphoma [2], adenocarcinoma not otherwise specified [1], lung and possibly lymphoma [1], and unspecified cancer [1]), 2 from cardiac events, and 1 from liver failure. The 2-year OS estimate was 91% (95% CI, 83%-99%), 5-year OS was 87% (95% CI, 77%-97%), and the 7-year OS estimate was 82% (Figure 1B).
Survival estimates. PFS (A) and OS (B) estimates of patients enrolled in SWOG S0313.
Survival estimates. PFS (A) and OS (B) estimates of patients enrolled in SWOG S0313.
Twenty-three patients with DLBCL were assigned GCB subtype based on immunohistochemistry, whereas 10 were non-GCB, and 11 had insufficient information. GCB and non-GCB subtypes had similar PFS and OS estimates.
Comparison with prior SWOG trials
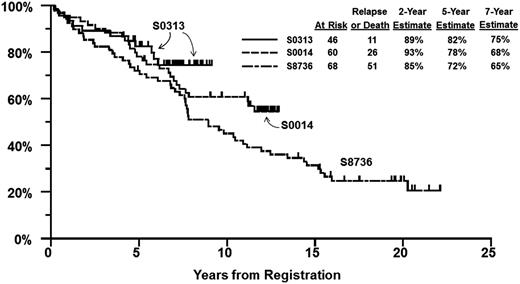
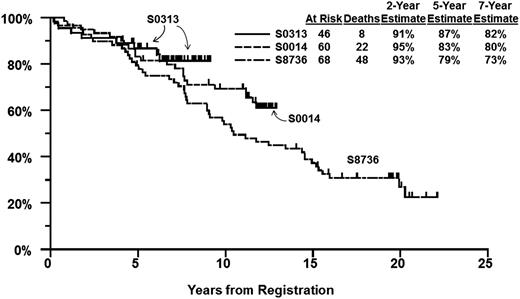
We identified 68 patients from SWOG study 8736 treated with CHOP(3) plus IFRT using the same eligibility criteria as for S0014 and S0313 (limited-stage disease with at least 1 stage-modified International Prognostic Index [IPI] adverse risk factor and aggressive B-cell lymphoma histology subtypes). The characteristics of patients on S8736, S0014 (historical control groups), and S0313 are shown in Table 2. S0313 patients were younger and more likely to be male, but were otherwise similar to matched controls from S0014 and S8736. PFS at 5 years was 72% (95% CI, 61%-83%) on S8736, 78% (95% CI, 68%-89%) on S0014, and 82% (95% CI, 71%-94%) on S0313 (Figure 2). For OS, the 5-year estimate was 79% (95% CI, 70%-89%) on S8736, 83% (95% CI, 74%-93%) on S0014, and 87% (95% CI, 77%-97%) on S0313 (Figure 3).
PFS by study. PFS estimates of patients enrolled in SWOG S0313 as compared with matched cohorts from prior studies S0014 and S8736.
PFS by study. PFS estimates of patients enrolled in SWOG S0313 as compared with matched cohorts from prior studies S0014 and S8736.
OS by study. OS estimates of patients enrolled in SWOG S0313 as compared with matched cohorts from prior studies S0014 and S8736.
OS by study. OS estimates of patients enrolled in SWOG S0313 as compared with matched cohorts from prior studies S0014 and S8736.
PFS according to study and risk group was analyzed for the 2 most recent studies. The 5-year (7-year) PFS in patients with a single stage-modified IPI risk factor was 85% (73%) in the historical control group S0014 vs 86% (78%) for S0313, and 5-year (7-year) PFS in patients with >1 risk factor was 61% (56%) in S0014 vs 75% (68%) in S0313.
Discussion
In our study, we describe that the addition of ibritumomab tiuxetan to CHOP(3) chemotherapy and IFRT produces excellent PFS and OS for patients with limited-stage aggressive B-cell NHL having high-risk features. The 2-year, 5-year, and 7-year estimates of outcome compare favorably to our prior published experience and to several other large studies. In the current study, 5-year PFS and OS were 82% and 87%, respectively. The historical 5-year PFS and OS for patients with limited-stage aggressive histologies of NHL, prior to the use of monoclonal antibodies, varied considerably by the number of adverse risk factors. PFS measured at 5 years has varied from 20% for patients having 3 or more risk factors to 70% for patients having 1 risk factor. Survival at 5 years has varied from 50% for patients with 3 or more risk factors to 77% for patients have only 1 adverse risk factor and, by 10 years, survival is dismal ranging from 0% to 50%.1-5 These benchmarks for comparison have been remarkably reproducible.
Current practice uses R-CHOP chemotherapy in advanced disease and we have reported the effect of adding rituximab to CHOP in limited-stage disease.6 In that study of 60 patients, eligibility criteria were identical to the current study and the treatment strategy parallel. Four doses of rituximab were combined with CHOP(3) and followed by IFRT. Five-year PFS and OS were 78% and 83%, respectively, and appear very similar to the current results. However, with longer follow-up, the relapses with ibritumomab tiuxetan consolidation appear to be fewer than with either of the prior trials, where 13% to 15% of patients relapsed or died between years 2 and 5 (vs 7% on S0313) and an additional 7% to 10% of patients relapsed or died between years 5 and 7 (vs 7% on S0313). Although direct statistical comparison cannot be made, there may be several possible explanations for this trend. It is possible that, as we hypothesized, RIT eliminated CD20-positive lymphocytes more efficiently than rituximab due to radioisotopes providing a cross-fire effect from the radiation exposure. However, patients on S0313 had a median age of 61 years as opposed to 69 years on S0014 and 66 on S8736, so competing death causes may not have affected them as much as patients on earlier trials. Differences in the length of follow-up could also have contributed to seeing fewer relapses on the most recent study, though with a median follow-up of 7.3 years, most relapses should have already occurred. Finally, it is also possible that 4 rituximab doses given with S0014 were insufficient to achieve an adequate anti-CD20 effect, particularly in males, who have faster rituximab clearance.9 The ongoing Intergroup trial, S1001, incorporates both rituximab with CHOP and ibritumomab tiuxetan consolidation following IFRT, and thus may help to answer the question of whether RIT provides additional value in limited-stage DLBCL.
One of the limitations of the current study is that it did not use rituximab in combination with CHOP, which was likely the main reason for slowing down patient accrual on the trial. This was due to preclinical and some clinical data indicating possible interference of rituximab with the efficacy of RIT.7,10 Another limitation is lack of staging with PET/CT scans, which was not routine at the time. Uniform staging with CT scans, however, makes the results of sequential SWOG studies comparable.
There were no unexpected toxicities with treatment. It is noteworthy that all but 4 patients completed therapy as prescribed (91%). Only 2 patients had therapy truncated because of toxicity. This low percentage contrasts with the reports of consolidation RIT after full-length treatment in advanced-stage DLBCL,11,12 where 30% of the patients were not able to complete RIT.
In the current study, only 4 patients (9%) had febrile neutropenia, as compared with 15% in our prior study, and over 20% in older patients receiving full-length treatment with R-CHOP. There have been no cases of treatment-related myeloid neoplasia with median 7.3 years of follow-up. This compares favorably with the data reported previously with ibritumomab tiuxetan, albeit in larger patient cohorts.13,14
Only 1 of 6 patients relapsed in the radiation field, with the in-field-only relapse rate of 16% to 18% remaining extremely consistent across prior SWOG, Eastern Cooperative Oncology Group (ECOG), and British Columbia Cancer Agency studies. This contrasts with 28% to 34% relapse rate seen in Groupe d’Etude des Lymphomes de l’Adulte (GELA) studies, where a number of patients did not receive radiation as prescribed, and the radiation was given later on average.15 Relapses do occur beyond year 5 (2 of 6) as previously reported. It is intriguing that the original site of involvement for all 6 patients who subsequently relapsed was in the head and neck area.
A full course of chemoimmunotherapy of 6 to 8 cycles without radiation has been used as an alternative to a short course of chemoimmunotherapy followed by radiation, based on low-risk disease trials such as the Mabthera International Trial.16 The 2 strategies cannot be compared directly in the absence of a randomized trial, leaving only cross-trial and retrospective comparisons. In the RICOVER-60 trial, the most favorable cohort of older patients with stage I nonbulky disease who received R-CHOP-14 × 6 had the 3-year event-free survival of 83.6%.17 This result is comparable to the current study, even though SWOG trials have consistently enrolled patients with bulky stage I disease, at least 1 adverse stage-modified IPI risk factor, and 20% to 23% of patients had systemic symptoms. The Osaka Lymphoma Study Group compared the 2 strategies retrospectively, and found the outcomes to be similar, although numerically favoring radiotherapy-containing treatment.18 Another retrospective study focused only on R-CHOP × 6 to 8 cycles, and found 5-year PFS of 84% and OS of 90% with an evidence of plateau.19 However, this study omitted the patients who required >20% dose reduction, thus selecting a more favorable cohort, and median follow-up of 4.3 years was still too short to capture potential relapses in limited-stage disease.
A very successful approach fusing response-adapted R-CHOP chemotherapy (4-6 cycles) followed by ibritumomab tiuxetan and if PET/CT-positive, by radiation, has been reported by ECOG. With a median follow-up of 4.3 years, the 4-year PFS rate was 88% and the OS rate was 98%, which was similar but numerically superior to the current study.20 We await publication with longer follow-up and risk stratification.
We continue to note a nearly constant rate of relapse and death for patients with early stages of DLBCL and that observation includes patients treated with CHOP(8) or CHOP(3), with monoclonal antibodies or without.4,6 We have hypothesized that limited-stage disease has a different biology than advanced-stage disease and have shown that of the highest 10 genes overexpressed in limited-stage disease, 7 were associated with host response.21 That finding is in contrast with advanced-stage disease, wherein all 10 of the highest expressed genes were associated with the lymphoma. We have also hypothesized that limited-stage DLBCL is more frequently of GCB origin,21 the finding that was recently corroborated in a retrospective study from the Memorial Sloan Kettering Cancer Center.22 In S0313, GCB vs non-GCB designation was limited by lack of adequate tissue, rendering us unable to use the Hans algorithm,23 but a less stringent definition also showed higher frequency of GCB and did not reveal significant differences in outcomes based on the designation. These observations are being tested in SWOG in both retrospective and prospective fashion.
We conclude that 3 cycles of CHOP and IFRT followed by ibritumomab tiuxetan consolidation is effective and safe therapy for patients with early-stage DLBCL having adverse-risk features. These results are similar to our prior experience with 4 infusions of rituximab added to 3 cycles of CHOP followed by IFRT, although the current trial has numerically superior 7-year PFS (75% vs 68%). Based upon our promising results, a current US Intergroup trial is testing R-CHOP and response-adapted involved field radiation, followed by consolidative radioimmunotherapy, in early-stage DLBCL.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients and their families, as well as all of the SWOG member institutions without whom this study would not be possible.
This work was supported in part by the following US Public Health Service Cooperative Agreement grant numbers awarded by the National Cancer Institute at the National Institutes of Health, US Department of Health and Human Services: CA32102, CA38926, CA11083, CA12612, CA46282, CA35431, CA35178, CA45807, CA20319, CA35192, CA04919, CA67663, CA12644, PO1 CA044991, R01 CA076287, and in part by IDEC Pharmaceuticals Corporation.
Authorship
Contribution: T.P.M. and R.I.F. designed the research; D.O.P., T.P.M., J.M.U., C.M.S., S.P., B.D.S., O.W.P., L.S.C., K.P.B., J.W.F., M.L., and R.I.F. performed the research; J.M.U. and M.L. performed statistical analyses; D.O.P., T.P.M., J.M.U., J.W.F., and R.I.F. analyzed and interpreted the data; D.O.P., T.P.M., and J.M.U. wrote the manuscript; and all authors reviewed the draft manuscript and approved the final version for submission.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Daniel O. Persky, University of Arizona Cancer Center, 1515 Campbell Ave, PO Box 245024, Tucson, AZ 85724-5024; email: dpersky@email.arizona.edu.
References
Author notes
D.O.P. and T.P.M. contributed equally to this manuscript.