Key Points
Combined R-bendamustine and ibrutinib is well tolerated in patients with relapsed/refractory NHL, with promising efficacy in MCL and FL.
Abstract
Ibrutinib has single agent activity of 22% to 68% in relapsed B-cell non-Hodgkin lymphoma(NHL). This study evaluated the safety and efficacy of ibrutinib combined with rituximab (R) and bendamustine. Patients received R (375 mg/m2) on day 1, bendamustine (90 mg/m2) on days 1 and 2, and ibrutinib (280 or 560 mg) on days 1 to 28 every 28 days for 6 cycles followed by ibrutinib alone until progression. Forty-eight patients enrolled, including 12 patients with follicular lymphoma (FL), 16 with diffuse large B-cell lymphoma (DLCL), and 17 with mantle cell lymphoma (MCL). No dose-limiting toxicities were observed. Patients received a median of 8 cycles, with 26 completing 6 cycles and continuing ibrutinib alone in cycles 7 to 34. The overall response (OR) rate was 72%, with 52% complete responses (CRs). By histology, the OR rate was 94% (76% CR) in MCL, 37% (31% CR) in DLCL, and 90% (50% CR) in FL. Grade 3 to 4 toxicities included lymphopenia (77%), neutropenia (33%), thrombocytopenia (19%), and rash (25%). Median progression-free survival has not been reached (95% CI, 8.7 months to not reached). The recommended phase 2 dose of ibrutinib in combination with R-bendamustine in patients with NHL is 560 mg. The combination has promising efficacy, particularly in MCL and FL. This trial was registered at www.clinicaltrials.gov as #NCT01479842.
Introduction
Ibrutinib is an oral inhibitor of Bruton’s tyrosine kinase, a protein critical to the B-cell receptor signaling cascade, with significant single agent activity in chronic lymphocytic leukemia/small lymphocytic lymphoma, mantle cell lymphoma (MCL), and the activated B-cell (ABC) subtype of diffuse large B-cell lymphoma (DLCL).1-4 In a phase 1 trial of ibrutinib in B-cell malignancies, grade 3 to 4 toxicity consisted of neutropenia (12.5%) and thrombocytopenia (7.2%), and overall response (OR) rate was 60%, with responses observed in patients with MCL, follicular lymphoma (FL), DLCL, marginal zone lymphoma (MZL), and Waldenstrom macroglobulinemia.3 Median progression-free survival (PFS) was 13.6 months. In a pivotal phase 2 trial in MCL,2 OR was 68%, with a complete response (CR) rate of 21% and an estimated median PFS of 13.9 months (95% CI, 7.0 to not reached). In a similar phase 2 trial in DLCL, the OR was 22%; however, responses were primarily restricted to patients with the ABC subtype of DLCL (OR 40% in ABC DLCL compared with 5% in germinal center B-cell [GCB] DLCL).4 PFS was 2.5 months in the patients with ABC DLCL.
Bendamustine combined with rituximab (R-bendamustine) is also an effective therapy for patients with newly diagnosed or relapsed/refractory B-cell non-Hodgkin lymphoma (NHL). In patients with relapsed indolent NHL and MCL, OR is 90% to 92% with 41% to 60% CRs and a median PFS of 23 to 24 months.5,6 Toxicities with R-bendamustine are predominantly hematologic, including grade 3 to 4 neutropenia (36%), thrombocytopenia (6%), febrile neutropenia (6%), and infection (10%). In the front-line setting for patients with indolent NHL or MCL, R-bendamustine is particularly active with OR of 93% to 97% and median PFS as long as 69 months.7,8 In patients with relapsed DLCL, R-bendamustine results in OR of 46% to 63% (CR 15% to 37%) with median PFS of 3.6 to 6.7 months.9,10 Based on the significant efficacy and tolerability of R-bendamustine in the front-line and relapsed settings, a number of recent trials have safely added novel agents including bortezomib and lenalidomide to R-bendamustine.11-13 Herein, we report the first phase 1/1b combination study of R-bendamustine and ibrutinib in patients with newly diagnosed MCL and relapsed or refractory indolent, MCL, and DLCL NHL.
Patients and methods
Study design and objective
This primary objective of this phase 1/1b, single center trial was to determine the dose-limiting toxicity (DLT), maximum tolerated dose (MTD), and toxicities with R-bendamustine combined with ibrutinib in patients with relapsed or refractory B-cell NHL. Once the MTD was defined, cohorts with 10 patients each with FL, DLCL, and MCL were planned.
The protocol was approved by the Ohio State University Institutional Review Board and conducted in accordance with the Declaration of Helsinki. The trial was registered at www.clinicaltrials.gov as #NCT01479842, and all patients provided written informed consent.
Patient eligibility
Patients at least 18 years of age with FL, MZL, WM, DLCL, transformed NHL, or MCL were eligible for the phase 1 trial. All patients were required to have received at least 1 prior therapy with the exception of patients with MCL. Prior therapy with bendamustine and R was permitted; prior ibrutinib was not. MCL patients were permitted to enroll at diagnosis provided they were not candidates or refused autologous stem cell transplantation (ASCT). Patients with DLCL had paraffin-embedded tissue centrally reviewed at Ohio State University and stained for CD10, bcl-6, and MUM-1 to assess cell of origin according to the Hans criteria.14
Additional eligibility criteria included Eastern Cooperative Group performance status of 0 to 2, 3 weeks from previous therapy, creatinine ≤2.0 mg/dL, bilirubin ≤1.5 × upper limit of normal, aspartate aminotransferase and alanine aminotransferase ≤2.5 × upper limit of normal, absolute neutrophil count ≥1000/µL, and platelet count ≥50 000/µL. Exclusion criteria included concurrent illness that would compromise study compliance or increase risk of toxicity, history of malabsorption, central nervous system lymphoma, HIV or hepatitis B or C, major surgery within 4 weeks of study enrollment, prior serious infusion reactions or hypersensitivity to R or bendamustine, and anticoagulation with warfarin, heparin, or low-molecular-weight heparin.
Treatment and evaluation
For the phase 1 trial, therapy consisted of R (375 mg/m2) on day 1 with bendamustine (90 mg/m2) on days 1 and 2 combined with 280 mg (dose level 1) or 560 mg (dose level 2) of ibrutinib on days 1 to 28. Cycles were 28 days, and combined R-bendamustine and ibrutinib was administered for 6 cycles. Responding patients could remain on ibrutinib alone after cycle 6 until disease progression.
On day 1 of a cycle, absolute neutrophil count ≥1000/µL and platelet counts ≥50 000/µL were required. Use of filgrastim or pegfilgrastim was permitted on day 3 of cycles 2 to 6 for grade 3 to 4 neutropenia on day 1 of a cycle or grade 4 neutropenia during a cycle. Dose reductions of bendamustine by 30 mg/m2 and ibrutinib by 140 mg were required for grade 3 to 4 neutropenia despite filgrastim or pegfilgrastim, grade 3 to 4 thrombocytopenia on day 1 of a cycle, grade 4 thrombocytopenia during a cycle, or any drug-related grade 3 to 4 nonhematologic toxicities. Dose reductions below 30 mg/m2 bendamustine or 140 mg ibrutinib were not permitted. Patients with DLT during cycle 1 or treatment delays >14 days were removed from the protocol.
Statistical analysis
Six patients were treated at dose levels 1 and 2, with the MTD defined as the highest dose level where ≤1 of 6 patients experienced DLT. DLT was defined during cycle 1 as any of the following events: grade 4 febrile neutropenia, treatment delays >14 days for hematologic or nonhematologic toxicity, or grade 4 nonhematologic toxicity. Once the MTD was determined, 10 patients each with FL, DLCL, and MCL were treated at the MTD or the recommended phase 2 dose (RP2D) if the MTD was not reached. In both the phase 1 and expansion cohorts, patients who did not complete 28 days of ibrutinib dosing during cycle 1 were replaced.
Toxicity was evaluated according to the Common Terminology Criteria for Adverse Events version 4.0. Response was assessed after cycles 3, 6, 9, 12, and 15 and then after every 6 cycles until disease progression according to International Harmonization response criteria for NHL.15 The maximum grade for each type of toxicity was recorded for each patient, and a frequency table was provided. PFS was defined from cycle 1 day 1 of study therapy until disease progression, start of another therapy, or death from any cause. If the patient did not have an event of disease progression or death, informative censoring occurred at the date of last follow-up. Overall survival (OS) was determined from the start of study therapy until death from any cause. Patients who were alive were censored at the date of last follow-up. Duration of response was calculated from the time of initial response (partial response [PR] or CR) until disease progression, start of another therapy, or death from any cause. Median duration of response, PFS, and OS were estimated according to the Kaplan-Meier method.
Results
Patient characteristics
From March 2012 until March 2014, 48 patients were enrolled, including 17 patients with MCL, 16 with DLCL, and 12 with FL. Median age was 62 (range, 23-84). For the patients with DLCL, 4 had GCB DLCL, 11 ABC DLCL, and 1 was unclassifiable by the Hans algorithm.14 Five of the 17 patients with MCL were previously untreated (ages 62-72). In the 43 previously treated patients, the median number of prior therapies was 3 (range, 1-10), 38% were refractory to their last therapy, 31% had an ASCT, and 13% had prior bendamustine. Additional patient characteristics are detailed in Table 1.
Patient characteristics, n = 48
| Characteristic . | No. . | % . |
|---|---|---|
| Median age (range) | 62 (23-84) | |
| Age ≥65 y | 20 | 42 |
| Sex | ||
| Male | 37 | 77 |
| Female | 11 | 23 |
| NHL subtype | ||
| FL | 12 | 25 |
| DLCL | 16 | 33 |
| DLCL, GCB type* | 4 | |
| DLCL, ABC type* | 11 | |
| DLCL, unclassifiable | 1 | |
| MCL | 17 | 35 |
| Transformed lymphoma | 2 | 4 |
| MZL | 1 | 2 |
| Number of prior therapies in 43 previously treated patients (range) | 3 (1-10) | |
| Number of previously untreated patients (MCL patients only) | 5 | |
| Refractory to last therapy | 18 | 38 |
| Prior ASCT | 15 | 31 |
| Prior bendamustine | 6 | 13 |
| Ann Arbor stage at study entry | ||
| I-II | 10 | 21 |
| III-IV | 38 | 79 |
| B-symptoms | 11 | 23 |
| Bone marrow involvement | 12 | 25 |
| Bulky disease | ||
| ≥5 cm | 20 | 42 |
| ≥10 cm | 3 | 6 |
| Prognostic score at study entry | ||
| IPI (n = 48) | ||
| 0-1, low risk | 16 | 33 |
| 2, low intermediate risk | 7 | 15 |
| 3, high intermediate risk | 16 | 33 |
| 4-5, high risk | 9 | 19 |
| FLIPI (n = 12) | ||
| 0-1, low risk | 4 | 33 |
| 2, intermediate risk | 5 | 42 |
| 3, poor risk | 3 | 25 |
| MIPI (n = 17) | ||
| <5.7, low risk | 7 | 41 |
| 5.7 to <6.2, intermediate risk | 4 | 24 |
| ≥6.2, high risk | 6 | 35 |
| Characteristic . | No. . | % . |
|---|---|---|
| Median age (range) | 62 (23-84) | |
| Age ≥65 y | 20 | 42 |
| Sex | ||
| Male | 37 | 77 |
| Female | 11 | 23 |
| NHL subtype | ||
| FL | 12 | 25 |
| DLCL | 16 | 33 |
| DLCL, GCB type* | 4 | |
| DLCL, ABC type* | 11 | |
| DLCL, unclassifiable | 1 | |
| MCL | 17 | 35 |
| Transformed lymphoma | 2 | 4 |
| MZL | 1 | 2 |
| Number of prior therapies in 43 previously treated patients (range) | 3 (1-10) | |
| Number of previously untreated patients (MCL patients only) | 5 | |
| Refractory to last therapy | 18 | 38 |
| Prior ASCT | 15 | 31 |
| Prior bendamustine | 6 | 13 |
| Ann Arbor stage at study entry | ||
| I-II | 10 | 21 |
| III-IV | 38 | 79 |
| B-symptoms | 11 | 23 |
| Bone marrow involvement | 12 | 25 |
| Bulky disease | ||
| ≥5 cm | 20 | 42 |
| ≥10 cm | 3 | 6 |
| Prognostic score at study entry | ||
| IPI (n = 48) | ||
| 0-1, low risk | 16 | 33 |
| 2, low intermediate risk | 7 | 15 |
| 3, high intermediate risk | 16 | 33 |
| 4-5, high risk | 9 | 19 |
| FLIPI (n = 12) | ||
| 0-1, low risk | 4 | 33 |
| 2, intermediate risk | 5 | 42 |
| 3, poor risk | 3 | 25 |
| MIPI (n = 17) | ||
| <5.7, low risk | 7 | 41 |
| 5.7 to <6.2, intermediate risk | 4 | 24 |
| ≥6.2, high risk | 6 | 35 |
FLIPI, follicular lymphoma international prognostic index; IPI, international prognostic index; MIPI, mantle cell lymphoma international prognostic index.
Based on immunohistochemical staining by the Hans algorithm.14
Treatment
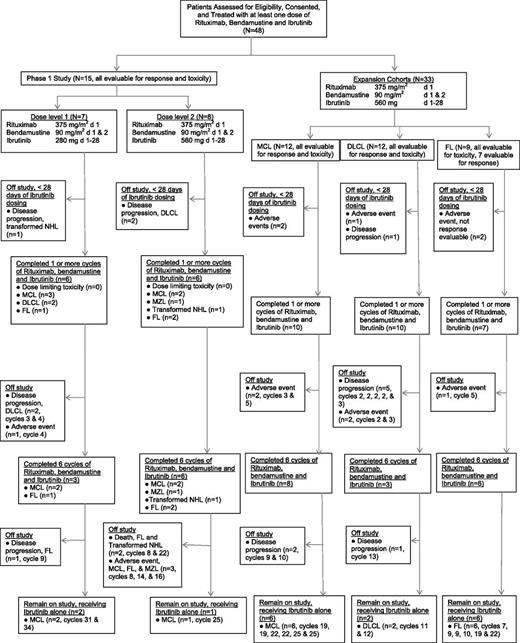
In the phase 1 study, 7 patients received R-bendamustine combined with 280 mg ibrutinib (dose level 1, see CONSORT diagram, Figure 1), with 1 patient with transformed NHL replaced for disease progression during cycle 1. In dose level 2 (560 mg ibrutinib), 8 patients were treated, with 2 patients with refractory DLCL replaced because of rapid disease progression. No DLTs were observed.
CONSORT diagram of the study. A total of 48 patients received study treatment, consisting at least of 1 dose of R, bendamustine, and ibrutinib. Thirty-nine patients completed 1 or more cycles of R, bendamustine, and ibrutinib, and 9 patients were removed from the study prior to completion of cycle 1 for rapid disease progression or adverse events.
CONSORT diagram of the study. A total of 48 patients received study treatment, consisting at least of 1 dose of R, bendamustine, and ibrutinib. Thirty-nine patients completed 1 or more cycles of R, bendamustine, and ibrutinib, and 9 patients were removed from the study prior to completion of cycle 1 for rapid disease progression or adverse events.
Thirty-three patients with MCL, DLCL, and FL were treated in planned expansion cohorts at the RP2D (Figure 1). The FL expansion cohort was closed prematurely because of limited drug availability. In the MCL expansion cohort, 12 patients were treated, and 2 previously untreated patients were replaced because of grade 3 neutropenia for >14 days and cellulitis with antibiotic-induced renal failure during cycle 1, respectively. In the DLCL cohort, 12 patients were treated, and 2 patients were replaced because of disease progression and grade 3 rash, respectively. In the FL cohort, 9 patients were treated, with 2 patients unable to complete cycle 1 because of grade 3 rash.
Patients completed a median of 8 cycles of therapy (range, 1-34), with 17 (35%) patients with FL (n = 6), MCL (n = 9), and DLCL (n = 2) still receiving study therapy at cycles 7 to 34. A total of 475 cycles have been administered. Twenty-six patients (9 FL, 12 MCL, 3 DLCL, 1 MZL, and 1 transformed NHL) completed 6 cycles of combined R-bendamustine and ibrutinib and continued to receive ibrutinib alone. A total of 31 patients have come off trial including 22 patients who stopped protocol therapy during cycles 1 to 5 because of progressive disease (n = 11), neutropenia >14 days (n = 5), fatigue (n = 1), thrombocytopenia >14 days (n = 1), rash (n = 3), and cellulitis with acute renal failure (n = 1). Nine additional patients came off study during cycles 8 to 22 for progressive disease (PD) (n = 4), rash (n = 1), recurrent nose bleeds (n = 1), neutropenia >14 days (n = 1), and death (n = 2).
Ten patients required pegfilgrastim. Dose modifications in the bendamustine to 60 mg/m2 were required in 3 patients for grade 3 neutropenia or thrombocytopenia. One patient required an additional bendamustine dose reduction to 30 mg/m2 during cycle 5 but, after the dose reduction, was able to complete all 6 cycles of combined therapy and currently is receiving ibrutinib alone in cycle 12. Ibrutinib dose reductions were required in 18 patients, including 4 patients with 2 dose reductions, for rash (n = 12), neutropenia (n = 3), thrombocytopenia (n = 4), hyperuricemia (n = 1), pancreatitis (n = 1), and atypical chest pain (n = 1).
Toxicities
For the 15 patients treated in the phase 1 study, grade 3 to 4 hematologic and nonhematologic adverse events during cycle 1 included lymphopenia (60%), neutropenia (20%), thrombocytopenia (7%), anemia (7%), and rash (7%). Grade 3 to 4 toxicities in all 48 patients across all cycles are listed by maximum grade in Table 2, with neutropenia (34%), lymphopenia (77%), thrombocytopenia (19%), and rash (25%) occurring most frequently. Febrile neutropenia occurred in 2 patients with pneumonia and enterocolitis, respectively. One responding patient with FL died of possible study-related toxicity. This patient developed acute respiratory distress syndrome (ARDS)/pneumonitis during cycle 8, with respiratory cultures from bronchoscopy specimens demonstrating only rhinovirus, and failed to recover despite broad-spectrum antibiotics and high-dose steroids.
Grade 3 to 5 adverse events
| . | Patients (N = 48) . | ||
|---|---|---|---|
| Grade 3, no. (%) . | Grade 4, no. (%) . | Grade 5, no. (%) . | |
| Hematologic toxicities | |||
| Neutropenia | 6 (13) | 10 (21) | |
| Lymphopenia | 16 (33) | 21 (44) | |
| Thrombocytopenia | 6 (13) | 3 (6) | |
| Anemia | 5 (10) | ||
| Febrile neutropenia* | 2 (4) | ||
| Nonhematologic toxicities | |||
| Rash | 12 (25) | ||
| Infection (nonneutropenic)† | 3 (6) | 1 (2) | 1 (2) |
| Nausea | 2 (4) | ||
| Vomiting | 1 (2) | ||
| Fever | 1 (2) | ||
| Hypotension | 2 (4) | ||
| Hypertension | 1 (2) | ||
| Hypokalemia | 1 (2) | ||
| Hyponatremia | 3 (6) | ||
| Hypophosphatemia | 3 (6) | ||
| Hyperuricemia | 1 (2) | ||
| Pancreatitis | 1 (2) | ||
| Fatigue | 1 (2) | ||
| . | Patients (N = 48) . | ||
|---|---|---|---|
| Grade 3, no. (%) . | Grade 4, no. (%) . | Grade 5, no. (%) . | |
| Hematologic toxicities | |||
| Neutropenia | 6 (13) | 10 (21) | |
| Lymphopenia | 16 (33) | 21 (44) | |
| Thrombocytopenia | 6 (13) | 3 (6) | |
| Anemia | 5 (10) | ||
| Febrile neutropenia* | 2 (4) | ||
| Nonhematologic toxicities | |||
| Rash | 12 (25) | ||
| Infection (nonneutropenic)† | 3 (6) | 1 (2) | 1 (2) |
| Nausea | 2 (4) | ||
| Vomiting | 1 (2) | ||
| Fever | 1 (2) | ||
| Hypotension | 2 (4) | ||
| Hypertension | 1 (2) | ||
| Hypokalemia | 1 (2) | ||
| Hyponatremia | 3 (6) | ||
| Hypophosphatemia | 3 (6) | ||
| Hyperuricemia | 1 (2) | ||
| Pancreatitis | 1 (2) | ||
| Fatigue | 1 (2) | ||
Febrile neutropenia included 1 case each of enterocolitis and pneumonia.
Infections include pneumonia (grade 3), cellulitis (grade 3), shingles (grade 3), cellulitis with sepsis (grade 4), and rhinovirus/ARDS (grade 5).
Rash was observed in 11/41 (27%) patients at the 560 mg dose and 1/7 (14%) patients receiving 280 mg ibrutinib. In 9 of 12 patients with grade 3 rash, the rash occurred in cycle 1 on days 8 to 15 as a diffuse macular-papular rash affecting trunk and limbs that was intensely pruritic. It typically resolved within 1 week after withdrawal of the ibrutinib and a short steroid course (<7 days). Three patients had rashes in cycles 3, 4, and 9. All but 4 patients were successfully rechallenged with ibrutinib at 1 lower dose level and full-dose R-bendamustine (375 mg/m2 and 90 mg/m2) without recurrence of the rash. In 3 patients (1 with DLCL and 2 with FL), diffuse erythroderma was observed within 4 to 6 hours of rechallenge with 420 mg ibrutinib during cycle 1 with associated transaminitis, fevers, hypotension, and eosinophilia. All 3 of these patients came off study but continued to receive full-dose R-bendamustine alone for 3 to 6 additional cycles. One patient had a lower-extremity ulcerative rash in cycle 4 that recurred in cycles 10 and 14 despite ibrutinib dose reductions leading to study removal. Skin biopsies in 2 patients revealed superficial perivascular dermatitis and subacute spongiotic dermatitis. Routine tumor lysis prophylaxis was not required on study, but 3 of 12 patients with a rash were receiving allopurinol. Two of these patients on allopurinol remained on it after ibrutinib dose reduction without recurrence of the rash, and 1 patient stopped allopurinol upon the first occurrence of the rash but still had diffuse erythroderma when re-treated with ibrutinib without allopurinol. Ten of 12 patients with a rash had either DLCL (n = 3) or FL (n = 7), with 1 patient each with MCL and MZL. Nine of 10 evaluable patients who developed a rash ultimately responded to R-bendamustine and ibrutinib.
Response and survival
Thirty-three of 46 evaluable patients responded for an OR of 72%, with 24 CRs (52%). Two patients with FL and a severe rash during cycle 1 were not evaluable as they continued on R-bendamustine alone without response assessment at study removal. OR by histology is summarized in Table 3. For the patients with DLCL, 5 CRs (31%) were observed in 3 patients with ABC-type DLCL, 1 with GCB DLCL, and 1 who was unclassifiable. Responses were observed across all dose levels of ibrutinib. Among 33 patients with a response, 8 patients have progressed (7 with CR and 1 with PR), and 2 have died. The median duration of response has not been reached (range, 2.8-28.0 months). The median time to response (CR or PR) was 2.8 months (range, 0.9-11.8 months), and the median time to CR was 2.9 months (range, 0.9-15.0 months).
Response by NHL subtype
| Histology . | No. evaluable patients* . | CR (%) . | PR (%) . | OR (%) . |
|---|---|---|---|---|
| MCL | 17 | 13 (76) | 3 (18) | 16 (94) |
| DLCL | 16 | 5† (31) | 1† (6) | 6 (37) |
| FL | 10* | 5 (50) | 4 (40) | 9 (90) |
| MZL | 1 | 0 | 1 (100) | 1 (100) |
| Transformed lymphoma | 2 | 1 (50) | 0 | 1 (50) |
| All patients | 46* | 24 (52) | 9 (20) | 33 (72) |
| Histology . | No. evaluable patients* . | CR (%) . | PR (%) . | OR (%) . |
|---|---|---|---|---|
| MCL | 17 | 13 (76) | 3 (18) | 16 (94) |
| DLCL | 16 | 5† (31) | 1† (6) | 6 (37) |
| FL | 10* | 5 (50) | 4 (40) | 9 (90) |
| MZL | 1 | 0 | 1 (100) | 1 (100) |
| Transformed lymphoma | 2 | 1 (50) | 0 | 1 (50) |
| All patients | 46* | 24 (52) | 9 (20) | 33 (72) |
Two patients with FL and grade 3 rash during cycle 1 were not evaluable.
Three patients with DLCL with a CR and 1 patient with a PR had ABC DLCL by the Hans immunohistochemical criteria.14 For the other 2 DLCL patients with a CR, 1 had GCB DLCL, and 1 was unclassifiable.
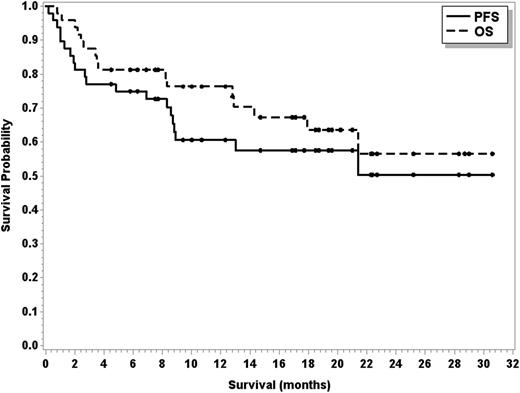
Sixteen patients have died of disease progression (n = 14), cardiovascular disease (n = 1), and ARDS/pneumonitis (n = 1). One responding patient with transformed MZL died during cycle 22 because of preexisting cardiovascular disease, and 1 responding patient with FL died because of ARDS/pneumonitis during cycle 8. Median PFS has not been reached (95% CI, 8.7 months to not reached), and median OS has also not been reached (95% CI, 17.9 months to not reached; see Figure 2). The estimated 2-year PFS and OS are 50.3% and 56.5%, respectively. For MCL and FL patients, the median PFS has not yet been reached, and for DLCL patients, the median PFS is 2.8 months (95% CI, 1.3-8.8 months). For patients achieving a CR and a PR, the median PFS has not been reached (95% CI, 21.4 months to not reached for CR; and 95% CI, 8.3 months to not reached for PR).
PFS (solid line) and OS (dashed line) in 48 patients treated with R, bendamustine, and ibrutinib.
PFS (solid line) and OS (dashed line) in 48 patients treated with R, bendamustine, and ibrutinib.
Discussion
This is the first trial to demonstrate the safety of ibrutinib combined with R-bendamustine in NHL. In this trial, 48 patients with relapsed or refractory NHL or newly diagnosed MCL received either 280 mg or 560 mg of ibrutinib with standard doses of R-bendamustine without DLT. OR was 72%, with responses observed in all NHL subtypes enrolled. Nearly all patients with MCL (94%) on this trial responded, and 76% achieved a CR, which compares favorably with the 68% OR (21% CR) reported with single agent ibrutinib2 and with the 75% to 92% OR (42% to 50% CR) with R-bendamustine in MCL.5,6 Therefore, this combination warrants further study in both the relapsed and front-line settings for patients with MCL and ideally should be compared in a front-line randomized trial to ibrutinib alone. The median duration of response with ibrutinib in MCL and the number of patients that convert to CR from PR after prolonged therapy is unclear at this point as a number of patients in the pivotal single agent trial2 and in the herein combination trial are continuing to receive ibrutinib at close to 3 years of treatment. A randomized study is necessary to determine if the higher CR rate with combined R-bendamustine and ibrutinib observed in this trial translates into a longer PFS than with ibrutinib alone.
In DLCL, the OR with R-bendamustine and ibrutinib was 37%, with 4 of the 6 responders having ABC-type DLCL according to the Hans algorithm. The OR and median PFS of 2.8 months is similar to the 40% OR and 2.5-month PFS in ABC-type DLCL reported by Wilson with single agent ibrutinib4 and suggests that the R-bendamustine is contributing very little in this relapsed/refractory patient population. Therefore, combinations with ibrutinib and chemotherapeutic agents with greater activity than R-bendamustine should be explored in patients with DLCL, and at least 1 such study with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (RCHOP) is ongoing.16 Lastly, the OR rate of 90% with CR of 50% in patients with relapsed FL in this combination study of R-bendamustine and ibrutinib appears superior to the 54.5% OR seen with single agent ibrutinib in FL.17 Although the OR in this trial is similar to the OR of 93% to 96% (54% to 71% CR) with R-bendamustine5,6 in patients with relapsed FL, the median PFS has not yet been determined, and further evaluation of this combination may be of interest in FL depending on the durability of response.
The incidence of grade 3 to 4 neutropenia (33%), lymphopenia (77%), and thrombocytopenia (19%) in this study is similar to that observed with R-bendamustine across multiple studies in patients with previously untreated7,8 and relapsed/refractory NHL.5,6,9,10 Surprisingly, infections were relatively uncommon in this trial even with prolonged use of ibrutinib and with the occurrence of grade 3 to 4 lymphopenia in the majority of patients. The 25% incidence of rash in our trial was unexpected. In previous single agent ibrutinib trials, the incidence of grade 3 to 4 rash was no greater than 2%, although grade 1 to 2 rashes were observed in 14% to 16% of patients.2-4 With R-bendamustine in patients with relapsed NHL, the reported incidence of grade 3 to 4 rash is low at 5%,9 with several studies reporting no grade 3 to 4 rash.5,6 However, in larger front-line studies, the occurrence of rash was as high as 16% to 24%.7,8 In other combination studies with ibrutinib, rash has been described, although less frequently. In a phase 1 trial of R-CHOP and ibrutinib,16 grade 1 to 2 rash was reported in 12% of patients, with no grade 3 to 4 events. In a phase 1 trial of R-bendamustine and ibrutinib in patients with chronic lymphocytic leukemia, grade 3 to 4 rash occurred in 10% of patients, although the doses of both ibrutinib and bendamustine were lower than in this trial in NHL, at 420 mg and 70 mg/m2, respectively.18 In this trial with R-bendamustine and ibrutinib in patients with relapsed NHL, rash typically occurred during cycle 1 between days 8 and 15, when the patients were receiving ibrutinib alone, and often resolved with a short course of steroids and withdrawal of the ibrutinib. Furthermore, in 11 patients who experienced the rash in cycles 1 to 6, all were re-treated with full-dose R-bendamustine without recurrence of the rash, including 3 patients who had significant rashes with rechallenge of ibrutinib during cycle 1 and came off study. Only 3 of the 12 patients with a rash were receiving concurrent allopurinol, and all but 1 patient remained on allopurinol after ibrutinib dose reduction without recurrent rash. This suggests that the ibrutinib is contributing to the rash and hypersensitivity reactions rather than the bendamustine or allopurinol. However, it is possible that the use of concomitant medications like allopurinol may increase the risk of dermatologic toxicity with ibrutinib, and this should be evaluated carefully in future studies. Lastly, although the numbers are small, it is possible that the occurrence of the rash is dose dependent and is predictive of early response.
It is important to note that although 10 responding patients required a <7-day course of steroids for rash, 23 other patients responded and received no additional steroids. Therefore, the responses are likely not related to steroids used in this trial to treat the rash. Based on the unexpected finding of grade 3 rash in this trial, we recommend that future ibrutinib combination studies incorporate a uniform management strategy for grade 3 to 4 rash with close attention to the role of concomitant medications in increasing the risk for this toxicity. In this study, 3 patients with hypersensitivity-type reactions after rechallenge with ibrutinib following a grade 3 rash were removed from the study and have not received further ibrutinib. We elected not to re-treat these patients with hypersensitivity reactions because of the rapidity and severity of symptoms, and if re-treatment in this situation was ever considered, close management in collaboration with dermatologists and allergists would be required.
In conclusion, the RP2D of R-bendamustine and ibrutinib in patients with relapsed or refractory NHL is R, 375 mg/m2; bendamustine, 90 mg/m2; and ibrutinib, 560 mg. Ibrutinib can also be continued safely beyond the initial 6 cycles of combined R-bendamustine and ibrutinib in responding patients. The promising toxicity and efficacy profile of this combination in patients with previously untreated and relapsed MCL, as well as relapsed FL, justifies larger phase 3 studies with this combination in these disease subtypes. However, as the OR rate with this combination in ABC-type DLCL is 36% (4 responses in 11 ABC DLCL patients) and is similar to the 40% OR rate reported with single agent ibrutinib in ABC DLCL,4 other combinations should be explored in this patient population. A multicenter, randomized phase 3 study comparing R-bendamustine to R-bendamustine and ibrutinib in patients 65 years of age or older with previously untreated MCL sponsored by Janssen Research and Development and Pharmacyclics is currently recruiting patients (www.clinical trials.gov, #NCT01776840). A similar randomized phase 3 international study of combined R-bendamustine or R-CHOP with ibrutinib for 6 cycles followed by ibrutinib until disease progression compared with R-CHOP or R-bendamustine alone for 6 cycles is also ongoing for patients with relapsed indolent NHL (www.clinical trials.gov, #NCT01974440). Special attention to the risk of rash and to early therapy discontinuation from prolonged cytopenias will be necessary in both of these trials. Given the selective activity of ibrutinib in ABC-type DLCL, ibrutinib with R-CHOP is being compared with R-CHOP alone as front-line therapy for ABC-type DLCL in combination with R-CHOP (www.clinicaltrials.gov, #NCT01855750). Lastly, a number of combination studies of ibrutinib with lenalidomide (www.clinicaltrials.gov, #NCT01955499 or #NCT01829568) are underway in an effort to develop patient friendly, long-term oral combination therapy for patients with lymphoma.
Presented in abstract form at the 54th annual meeting of the American Society of Hematology, Atlanta, GA, December 8, 2012.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by Pharmacyclics (K.A.B., K.M., S.J., J.A.J., J.C.B.).
Authorship
Contribution: K.A.B. and J.C.B. were responsible for conception and design; K.M., B.C., S.J., J.F., J.A.J., and G.L. provided study materials or patients; K.A.B., K.M., C.J., G.L., and L.W. performed collection and assembly of data; K.A.B. and L.W. performed data analysis and interpretation; K.A.B. wrote the manuscript; and all authors approved the final manuscript.
Conflict-of-interest disclosure: K.A.B., B.C., K.M., S.J., J.A.J., and J.C.B. receive research funding from Pharmacyclics. J.C.B. has an unpaid advisory/consultant role with Pharmacyclics. The remaining authors declare no competing financial interests.
Correspondence: Kristie A. Blum, The Ohio State University, B315 Starling Loving Hall, 320 West 10th Ave, Columbus, OH 43210; e-mail: kristie.blum@osumc.edu.