Key Points
Initial imatinib-based therapy of Ph+ adult ALL is associated with lower early mortality and higher CR rate.
In adults with Ph+ ALL, allogeneic SCT in first CR prolongs relapse-free survival and OS.
Abstract
In this study, we randomly compared high doses of the tyrosine kinase inhibitor imatinib combined with reduced-intensity chemotherapy (arm A) to standard imatinib/hyperCVAD (cyclophosphamide/vincristine/doxorubicin/dexamethasone) therapy (arm B) in 268 adults (median age, 47 years) with Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia (ALL). The primary objective was the major molecular response (MMolR) rate after cycle 2, patients being then eligible for allogeneic stem cell transplantation (SCT) if they had a donor, or autologous SCT if in MMolR and no donor. With fewer induction deaths, the complete remission (CR) rate was higher in arm A than in arm B (98% vs 91%; P = .006), whereas the MMolR rate was similar in both arms (66% vs 64%). With a median follow-up of 4.8 years, 5-year event-free survival and overall survival (OS) rates were estimated at 37.1% and 45.6%, respectively, without difference between the arms. Allogeneic transplantation was associated with a significant benefit in relapse-free survival (hazard ratio [HR], 0.69; P = .036) and OS (HR, 0.64; P = .02), with initial white blood cell count being the only factor significantly interacting with this SCT effect. In patients achieving MMolR, outcome was similar after autologous and allogeneic transplantation. This study validates an induction regimen combining reduced-intensity chemotherapy and imatinib in Ph+ ALL adult patients and suggests that SCT in first CR is still a good option for Ph+ ALL adult patients. This trial was registered at www.clinicaltrials.gov as #NCT00327678.
Introduction
The Philadelphia chromosome (Ph) derives from the balanced t(9;22)(q34;q11.2) chromosomal translocation, resulting in the BCR-ABL1 fusion gene encoding an oncoprotein with constitutive tyrosine kinase activity and abnormal cytoplasmic localization.1 Ph-positive (Ph+) acute lymphoblastic leukemia (ALL) represents 25% to 30% of adult ALL, and its incidence increases with age. Two types of fusion protein are found, resulting from a different breakpoint cluster region (bcr) in the BCR gene. In Ph+ ALL, the p190-BCR-ABL1 (minor [m]-bcr) subtype is more frequent than the p210-BCR-ABL1 (major [M]-bcr) subtype, commonly found in chronic myeloid leukemia.2,3 In the era before tyrosine kinase inhibitors (TKIs), Ph positivity conferred a bad prognosis to ALL patients, with long-term survival rates <20%.4-8 For these patients, allogeneic hematopoietic stem cell transplantation (SCT) was therefore considered the only potential curative option.
The advent of TKIs targeting BCR-ABL1, the first being imatinib, led to major changes in the outcome of Ph+ ALL patients. Treatment with TKI/chemotherapy combinations yielded very high complete remission (CR) rates and 2-year overall survival (OS) rates of ∼60%.9-13 Updating the results of our first combined Group for Research on Adult Acute Lymphoblastic Leukemia Philadelphia positive(GRAAPH)-2003 study, we recently reported a 52% OS rate at 4 years.14 Many questions remained, however, on how to optimize the combination of TKIs and chemotherapy. The role of SCT in first CR (CR1) also needed to be confirmed in this new context. We therefore initiated a randomized study comparing 2 strategies during the first induction cycle, one with less-intense chemotherapy combined with imatinib over the entire induction period, and the other with the more intense regimen hyperCVAD (cyclophosphamide/vincristine/doxorubicin/dexamethasone) and imatinib given only for the first 2 weeks. The aim was to decrease toxicity without increasing the risk of relapse. We used early BCR-ABL1 minimal residual disease (MRD) levels as a surrogate end point.
Patients and methods
Study design
The GRAAPH-2005 study was conducted in 60 centers in France and Switzerland. Patients aged 18 to 59 years with newly diagnosed Ph+ and/or BCR-ABL1–positive ALL were eligible. Patients with known chronic myeloid leukemia in blastic phase; cardiac disease; renal or hepatic dysfunction (ie, serum creatinine level >2 upper limit of normal [ULN], bilirubin level >2 ULN, aspartate aminotransferase level >1.5 ULN, or alanine aminotransferase level >2.5 ULN); HIV, human T-lymphotropic virus, hepatitis B virus, or hepatitis C virus infection; contraindication to intensive chemotherapy; or pregnancy were not eligible. Written informed consent was obtained from all patients. The study was approved by the Institutional Ethics Committee Ile-de-France VI, France, and conducted in accordance with the Declaration of Helsinki. Between May 2006 and August 2011, 270 consecutive patients entered the study. Because 1 patient was lost to follow-up and 1 patient withdrew consent, the evaluation population totaled 268 patients. A patient flowchart is provided in the supplemental Appendix, available on the Blood Web site.
Diagnosis of Ph+ ALL and MRD monitoring
Ph positivity was determined during the prephase by standard karyotype and/or fluorescence in situ hybridization analysis and/or BCR-ABL1 fusion transcript detection with quantitative reverse-transcription polymerase chain reaction (qRT-PCR), centralized in 3 laboratories that used standardized methods with international scale. BCR-ABL1 transcript levels were used to monitor MRD. Major molecular response (MMolR) was defined as a BCR-ABL1/ABL ratio of ≤0.1% in the bone marrow, and molecular CR was defined by the absence of detectable MRD with a sensitivity of at least 0.01%. The primary end point was the MMolR rate after cycle 2.
Treatments
Treatments are shown in Table 1 (detailed in the supplemental Appendix). Response was assessed at day 29 of cycles 1 and 2 and evaluated by conventional morphologic criteria together with bone marrow MRD evaluation. Hematologic CR was defined as <5% marrow blasts with adequate blood count recovery. Patients aged ≤55 years were eligible for allogeneic SCT in CR1 if they had a donor (HLA-identical sibling or 10/10 or 9/10 HLA-compatible unrelated donors). On May 23, 2007, the protocol was amended to allow reduced-intensity conditioning (RIC) in patients >55 years old or presenting a contraindication to myeloablative conditioning (MAC), making all patients eligible for allogeneic SCT. This amendment was effective on June 28, 2007, after enrollment of the first 63 patients. Patients in MMolR after cycle 2 but without donor (or >55 years old prior to the RIC amendment) were eligible for autologous SCT using the same MAC. Maintenance therapy was planned after autologous SCT, whereas no systematic maintenance was planned after allogeneic SCT. Patients who failed to achieve MMolR after cycle 2 were further treated with imatinib combined with chemotherapy according to the more intense cyclophosphamide/vincristine/doxorubicin/dexamethasone protocol (hyperCVAD).15
Statistical methods
Assuming a 45% MMolR rate in arm B, the sample size was calculated at 270 patients to demonstrate the noninferiority in MMolR rate in arm A, for an α error of 0.05 and a power of 80%. Noninferiority was defined as MMolR rate equal to 15% worse (δ = −0.15) and tested with the likelihood score test of Farrington and Manning.16 A logistic regression model was used to assess the impact of covariates on MMolR and molecular CR. Secondary end points were event-free survival (EFS), relapse-free survival (RFS), cumulative incidence of relapse (CIR), cumulative incidence of nonrelapse-related mortality (NRM), and OS. Molecular relapse or persistence was not considered an RFS or CIR event. The Kaplan-Meier method was used to estimate EFS, RFS, and OS probabilities.17 When evaluating CIR and NRM, estimations took into account deaths in CR1 and hematologic relapses, respectively, as competing events. Outcome comparisons were performed by Cox models.18 The effect of SCT in CR1 was analyzed by the time-dependent Mantel-Byar method and graphically illustrated by Simon and Makuch plots,19,20 t0 being the time of hematologic or molecular response assessment (supplemental Appendix). Outcome comparisons were performed by Andersen-Gill models.21 For testing the differential effects of SCT in patient subgroups, interaction terms were included in the model. We also performed a landmark standard donor vs no-donor analysis, which is presented in the supplemental Appendix. Binary variable comparisons were performed using Fisher’s exact test. Median comparisons were performed by the Mann-Whitney 2-sample test. Type 1 error was fixed at the 5% level. All tests were 2-tailed. Hazard ratios (HRs) are given with 95% confidence intervals (CIs). Statistical analyses were performed with the STATA/IC 12.1 software package (StataCorp, College Station, TX).
Results
Patient characteristics
Patient characteristics were well balanced between randomization arms, except for gender, with more men in arm B (Table 2). Median age was 47 years old (range 21-60). Karyotype/fluorescence in situ hybridization analysis was performed in all patients but failed in 15. Ph positivity was evidenced in 247 patients, 6 having either a normal karyotype or an isolated trisomy 11 (1 patient). Bcr analysis was available in 267 patients, with 195 m-bcr fusions, 69 M-bcr fusions, and 3 patients having both subtypes. ACAs were observed in 176 patients, more frequently in m-bcr than in M-bcr cases (74% vs 58%; P = .013). No significant differences in age and WBC were noted in patients with M-bcr or ACAs.
Initial therapy: response, toxicity, and compliance
Initial response is shown on Table 3. Due to fewer induction deaths, the hematologic CR rate was higher in arm A (98.5% vs 91.0% in arm B; P = .006). Among the 254 patients alive in CR after cycle 2, 205 (81%) were tested for MRD level. The characteristics of these patients did not differ from those of the 49 nontested patients, with the exception of more frequent M-bcr–positive MRD in tested patients (supplemental Table 1). After cycle 2 (MRD2 time point), 134 patients (65.4%) reached MMolR, including 53 patients (25.8%) in molecular CR with undetectable transcript. At MRD2, the MMolR rate was similar in both randomization arms (66.1% vs 64.5% in arms A and B, respectively; P = .88) and, with respect to this primary end point, the noninferiority of arm A was demonstrated (δ 95% CI, −0.126 to 0.093; P = .006).
Achievement of MMolR at MRD2 was more frequent in patients with a lower initial WBC as well as in those with m-bcr ALL. In m-bcr patients, respective MMolR and molecular CR rates were 74% and 32% vs 43% and 9% in M-bcr patients (P < .001 and P = .001, respectively). After adjustment, m-bcr and lower WBC remained independently predictive of a good MRD2 response. MMolR and molecular CR rates were 83% and 34%, 62% and 23%, 53% and 10%, and 31% and 8% in patients with m-bcr and WBC <30 × 109/L, m-bcr and WBC ≥30 × 109/L, M-bcr and WBC <30 × 109/L, and M-bcr and WBC ≥30 × 109/L, respectively. Neither age nor the presence of ACAs significantly impacted MRD2 response.
Hematologic and grade 3/4 toxicities observed during the first 2 cycles are shown in supplemental Table 2). As anticipated, hematologic toxicity was lower in arm A during the first cycle, associated with a lower incidence of infections. Surprisingly, the reverse was observed during the second cycle, which was similar in both arms.
Patients alive after cycle 1 received a median imatinib dose (calculated as the total dose received by the patient divided by the theoretical number of days, as stated by the protocol) of 800 mg/d (range, 28-1025 mg/d) for a median of 28 days (range, 1-35 days) in arm A and 800 mg/d (range, 371-1600 mg/d) for a median of 14 days (range, 7-28 days) in arm B (P = .28). During cycle 2, the median dose was also 800 mg/d (range, 0-1257 mg/d) for a median of 14 days (range, 0-22 days) and 800 mg/d (range, 160-1371 mg/d) for a median of 14 days (range, 3-24 days), in arm A and arm B, respectively (P = .43). During the pretransplant interphase, the median dose was 600 mg/d in both arms (range, 300-1428 mg/d) for a median of 14 days (range, 7-33 days). Reasons for not receiving the planned imatinib dose were mostly related to toxic adverse events. After autologous SCT (n = 35 patients), 12 patients received the planned 12 cycles of imatinib, 3 patients did not receive any imatinib due to early relapse, and the others received between 1 and 11 imatinib cycles, interruptions being mostly due to relapse.
Outcome by randomization arm
At a median follow-up of 4.8 years, 140 patients have died. Among the 254 CR patients (133 in arm A, 121 in arm B), 92 relapsed (43 in arm A, 49 in arm B) and 128 died (66 in arm A, 62 in arm B), including 58 deaths in CR1 (31 in arm A, 27 in arm B). Median EFS and OS were 2.1 years and 3.6 years, respectively. At 5 years, the EFS rate was estimated at 37.1% (95% CI, 31.1-43.1) and the OS rate at 45.6% (95% CI, 39.2-51.8). As illustrated in Figure 1, patients randomized in arm A tended to have a longer EFS (median, 2.5 vs 1.8 years; HR, 1.27 [95% CI, 0.93-1.72]; P = .13) and OS (median, 4.1 vs 3.3 years; HR, 1.17 [95% CI, 0.84-1.62]; P = .37) than patients randomized in arm B. After CR was achieved, CIR was nonsignificantly higher in arm B (41.3% [95% CI, 33.0-50.8] vs 32.8% [95% CI, 25.4-41.5] in arm A at 5 years; P = .34), whereas NRM was comparable in both arms (22.6% [95% CI, 16.1-31.2] in arm B vs 23.7% [95% CI, 17.3-32.0] in arm A at 5 years; P = .90). The longer EFS observed in arm A became more apparent and statistically significant when focusing on the 229 patients ≥30 years old (HR, 1.43 [95% CI, 1.03-1.98]; P = .034), even if this did not translate into longer OS (HR, 1.28 [95% CI, 0.90-1.83]; P = .17).
Outcome by randomization arm. (A) EFS by randomization arm. At 5 years, the EFS rate was estimated at 32.1% (95% CI, 24.0-40.4) in arm B vs 42.2% (95% CI, 33.5-50.6) in arm A (P = .13). (B) OS by randomization arm. At 5 years, the OS rate was estimated at 43.0% (95% CI, 33.9-51.7) in arm B vs 48.3% (95% CI, 39.2-56.8) in arm A (P = .37).
Outcome by randomization arm. (A) EFS by randomization arm. At 5 years, the EFS rate was estimated at 32.1% (95% CI, 24.0-40.4) in arm B vs 42.2% (95% CI, 33.5-50.6) in arm A (P = .13). (B) OS by randomization arm. At 5 years, the OS rate was estimated at 43.0% (95% CI, 33.9-51.7) in arm B vs 48.3% (95% CI, 39.2-56.8) in arm A (P = .37).
SCT cohorts
Among the 254 CR patients, 148 were transplanted in CR1 with a donor identified by protocol criteria, including 76 sibling and 72 unrelated donors (Table 4). Thirteen additional patients received cord blood (CB) transplantation, not planned by the protocol, leading to a total of 161 patients in the allogeneic SCT cohort (63% of the CR population). Thirty-seven of them received RIC-SCT, including 14 patients <55 years old. As expected, patients receiving RIC were significantly older than those receiving MAC (median, 56.2 years [range, 30-59 years] vs 39.8 years [range, 18-57 years]; P < .001). The no–allogeneic SCT cohort consisted of the remaining 93 CR patients. Primary reasons for not receiving allograft were as follows: age >55 years before the RIC amendment (n = 7), early relapse (n = 15) or early death in CR (n = 6), baseline or acquired contraindication for SCT (n = 8), no identified donor (n = 49), investigator choice (n = 4), patient refusal (n = 1), and unknown (n = 3). The rate of patients who did not achieve MMolR at the MRD2 time point was similar in the allogeneic SCT and no–allogeneic SCT cohorts (33% and 38%, respectively; P = .54). As planned by the protocol, no patient received preemptive TKI maintenance after allogeneic SCT. Patients were monitored for posttransplant MRD levels, and reintroduction of TKIs was driven by MRD. A total of 38 patients eventually received posttransplant TKIs (18 imatinib, 20 dasatinib) for molecular relapse/persistence, 19 in each arm.
Among the 93 nonallografted patients, 39 were thus eligible for autologous SCT because they were in MMolR at MRD2 and had no donor, and 28 of them were actually autografted in CR1. Seven additional patients received autologous SCT, including 6 patients with poor or unknown MRD2 level and a 59-year-old patient with a sibling donor and good MRD2 response, leading to a total of 35 patients in the autologous SCT cohort (14% of the CR population). As expected, more patients were in MMolR at MRD2 in the autologous cohort than in the allogeneic SCT cohort (83% vs 56%; P = .004). In the remaining 58 CR patients, primary reasons for not receiving autologous SCT were as follows: noneligibility due to poor or unknown MRD2 level (n = 43), early relapse (n = 7), baseline or acquired contraindication for SCT (n = 4), investigator choice (n = 1), patient refusal (n = 1), and unknown (n = 2). All 35 patients who received autologous SCT initiated the planned post-SCT maintenance, except for 3 patients due to early relapse.
Posttransplant outcome
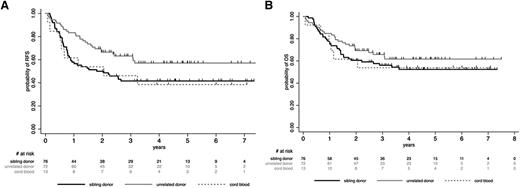
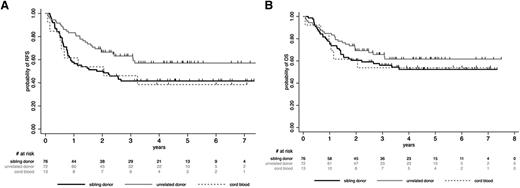
Patients who received allogeneic SCT from unrelated donors tended to have a better outcome than those transplanted from sibling donors or CB, but these differences were not statistically significant (supplemental Appendix; Figure 2). For further analyses, we thus considered all these patients (76 sibling donors, 72 unrelated donors, 13 CB) in a single allogeneic cohort. In this cohort, 5-year posttransplant RFS and OS rates were 48.3% (95% CI, 40.2-56.0) and 56.7% (95% CI, 48.4-64.2), respectively. CIR was 25.4% (95% CI, 19.3-33.0), whereas NRM was 25.8% (95% CI, 19.7-33.4). Posttransplant outcome according to the conditioning regimen (RIC vs MAC) is detailed in supplemental Table 3. Interestingly, MRD2 response did not influence post–allogeneic SCT outcome, as illustrated for RFS in supplemental Figure 1. In the autologous cohort, posttransplant RFS, OS, CIR, and NRM rates were 46.1% (95% CI, 28.3-62.1), 55.1% (95% CI, 35.5-70.9), 47.5% (95% CI, 32.1-65.7), and 6.1% (95% CI, 1.5-22.2), respectively. In both allogeneic and autologous cohorts, no differences in posttransplant outcome were observed between randomization arms (supplemental Table 4). Unexpectedly, trends toward higher CIR and lower NRM were nonetheless observed after allogeneic SCT in the more chemointensive arm B as compared with the imatinib-based arm A.
Post-SCT outcome by stem cell source (allogeneic SCT cohort). (A) Post-SCT RFS by stem cell source. At 5 years, the posttransplant RFS rate was 57.1% (95% CI, 44.1-68.2) in patients who received SCT from unrelated donors, 41.6% (95% CI, 30.0-52.4) in those who received SCT from sibling donors, and 38.5% (95% CI, 14.0-62.8) in those who received CB-SCT (P = .22). (B) Post-SCT OS by stem cell source. At 5 years, the posttransplant OS rate was 61.7% (95% CI, 48.7-72.3) in patients who received SCT from unrelated donors, 52.3% (95% CI, 40.1-63.2) in those who received SCT from sibling donors, and 53.9% (95% CI, 24.8-76.0) in those who received CB-SCT (P = .52).
Post-SCT outcome by stem cell source (allogeneic SCT cohort). (A) Post-SCT RFS by stem cell source. At 5 years, the posttransplant RFS rate was 57.1% (95% CI, 44.1-68.2) in patients who received SCT from unrelated donors, 41.6% (95% CI, 30.0-52.4) in those who received SCT from sibling donors, and 38.5% (95% CI, 14.0-62.8) in those who received CB-SCT (P = .22). (B) Post-SCT OS by stem cell source. At 5 years, the posttransplant OS rate was 61.7% (95% CI, 48.7-72.3) in patients who received SCT from unrelated donors, 52.3% (95% CI, 40.1-63.2) in those who received SCT from sibling donors, and 53.9% (95% CI, 24.8-76.0) in those who received CB-SCT (P = .52).
Role of SCT in CR1
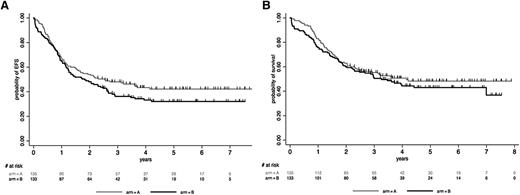
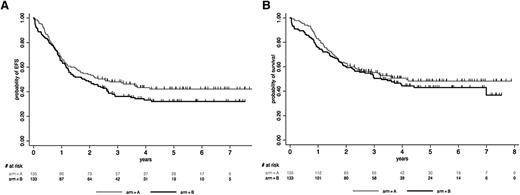
We first analyzed the impact of allogeneic SCT in the whole population of 254 CR patients. We included the 13 CR patients >55 years old and enrolled before the RIC amendment in this comparison, because 6 of them had received RIC-SCT. As illustrated in Figure 3A-B, allogeneic SCT in CR1 was associated with a significant benefit in RFS (HR, 0.69 [95% CI, 0.49-0.98]; P = .036) and OS (HR, 0.64 [95% CI, 0.44-0.93]; P = .02). A higher WBC was the only factor identified as interacting with this beneficial effect (P = .007 and P = .033 for RFS and OS interactions, respectively). This finding is illustrated in supplemental Figure 2, which shows that only patients with a high WBC significantly benefited from SCT when using 30 × 109/L as a WBC cutoff. We also observed that patients achieving a molecular CR at MRD2 did not benefit from SCT in term of RFS (HR, 1.02 [95% CI, 0.47-2.21]; P = .96), whereas those with persistent MRD did (HR, 0.62 [95% CI, 0.40-0.96]; P = .034), even if interaction was not here statistically significant (P = .18) (supplemental Figure 3). We also did a donor vs no-donor analysis (Table 5; supplemental Appendix). Using this methodology, RFS and OS were not significantly improved in the donor group. Repeating this analysis after excluding the 13 patients who received unplanned CB-SCT from the no-donor group yielded similar results for RFS and OS (HR, 0.74 [95% CI, 0.51-1.08; P = .11] and 0.71 [95% CI, 0.48-1.06; P = .10], respectively).
Role of SCT in CR1. Simon-Makuch plots for RFS (A) and OS (B) in CR patients. t0 was the time of hematologic CR achievement. RFS and OS were significantly prolonged in the allogeneic SCT cohort (HR, 0.69 [95% CI, 0.49-0.98; P = .036] and 0.64 [95% CI, 0.44-0.93; P = .020], respectively, by the Andersen-Gill test). Simon-Makuch plots for RFS (C) and OS (D) in patients in MMolR at MRD2 time point. t0 was the time of MRD2 MMolR achievement. RFS and OS were similar in the autologous and allogeneic SCT cohorts (HR, 0.94 [95% CI, 0.53-1.65; P = .82] and 0.95 [95% CI, 0.51-1.74; P = .86], respectively, by the Andersen-Gill test). A 3-month RFS landmark period (median time from CR to transplantation) was used, because patients should be alive but also in CR1 to be actually transplanted. This landmark allowed minimizing the bias related to early relapses when comparing OS with this method.
Role of SCT in CR1. Simon-Makuch plots for RFS (A) and OS (B) in CR patients. t0 was the time of hematologic CR achievement. RFS and OS were significantly prolonged in the allogeneic SCT cohort (HR, 0.69 [95% CI, 0.49-0.98; P = .036] and 0.64 [95% CI, 0.44-0.93; P = .020], respectively, by the Andersen-Gill test). Simon-Makuch plots for RFS (C) and OS (D) in patients in MMolR at MRD2 time point. t0 was the time of MRD2 MMolR achievement. RFS and OS were similar in the autologous and allogeneic SCT cohorts (HR, 0.94 [95% CI, 0.53-1.65; P = .82] and 0.95 [95% CI, 0.51-1.74; P = .86], respectively, by the Andersen-Gill test). A 3-month RFS landmark period (median time from CR to transplantation) was used, because patients should be alive but also in CR1 to be actually transplanted. This landmark allowed minimizing the bias related to early relapses when comparing OS with this method.
We then compared the outcome of patients who received allogeneic SCT vs autologous SCT, restricting the comparison to the 134 patients who were in MMolR at MRD2 because it was an eligibility criterion for autologous transplantation. Among these patients, 90 were allografted and 29 were autografted in CR1. As shown in Figure 3C-D, RFS and OS did not differ after autologous or allogeneic SCT in these patients (HR, 0.94 [95% CI, 0.53-1.65; P = .82] and 0.95 [95% CI, 0.51-1.74; P = .86], respectively). Similar RFS and OS results were observed when patients who received RIC-SCT were excluded from the allogeneic cohort (HR, 1.15 [95% CI, 0.63-2.10; P = .64] and 1.02 [95% CI, 0.54-1.93; P = .95], respectively).
Multivariable analysis
In univariable analysis, increasing age and body mass index (BMI) were associated with a worse EFS and OS. Neither WBC, bcr subtype, presence of ACAs, nor early MRD response significantly influenced the outcome. In a multivariable analysis that included treatment arm, allogeneic SCT as a time-dependent variable, age and BMI as continuous variables, WBC using the 30 × 109/L cutoff, and a WBC/SCT interaction term because of the significant interaction mentioned above, allogeneic SCT in CR1 and WBC <30 × 109/L remained the 2 factors independently associated with longer RFS (HR, 0.56 [95% CI, 0.35-0.91; P = .019] and 0.56 [95% CI, 0.34-0.94; P = .029], respectively).
Discussion
This study is the first large randomized study reporting the long-term outcome of adult patients with Ph+ ALL treated with a combined TKI/chemotherapy strategy. It confirms previous reports that such a combined treatment yields very high CR rates and higher proportions of patients receiving SCT in CR1, resulting in improved long-term survival.9-13,22 However, relapses still occur and, despite the introduction of RIC in older patients, allogeneic SCT is still associated with a substantial NRM in these patients, leading to an overall outcome similar to, but not better than, that observed in adults with Ph-negative ALL.
The aim of the study was to investigate whether an initial treatment based on imatinib combined with RIC might lower early toxicity and, eventually, posttransplant NRM, without decreasing the early molecular response rate. Overall, 77% of the patients could be brought to SCT (63% allogeneic, 14% autologous), which is higher than the percentage observed in the preimatinib era or in some other reports.19-21 Results turned out to be positive on the short-term, validating the concept of initial TKI-based therapy as associated with lower early mortality and higher CR rate with comparable MRD response rate. This may be related to the longer exposure to TKI in arm A (6 weeks compared to 4 weeks in arm B) as shown in previous studies reporting that continuing dosing of imatinib was associated with better outcome.23,24 However, post–allogeneic SCT outcome was similar in both randomization arms, leading to similar and still unsatisfactory EFS and OS. Together, our approach nonetheless yielded 5-year EFS and OS rates of 37.1% and 45.6%, respectively, which are at least comparable to the recent UKALLXII/ECOG2993 study reporting 33% EFS and 38% OS rates at 4 years, the Northern Italy Leukemia Study Group showing 23% EFS and 38% OS rates at 5 years, and the GRAAPH-2003 pilot study.14,22,25
The role of allogeneic transplantation in CR1 in Ph+ ALL adults has been recently discussed, with the emerging hypothesis that the use of TKIs frontline and during the entire therapy might improve the outcome of these patients enough that allogeneic SCT might not be necessary in CR1.9,26 Our study, however, suggests that allogeneic SCT remains the best current postremission option in younger patients able to tolerate this strategy. In a time-dependent analysis, this strategy was associated with prolonged RFS and OS, even when the donor vs no-donor comparison did not reach statistical significance (Table 5). However, the NRM was as high as 25% in transplanted patients, even when RIC was introduced early during the trial course. This high NRM was likely related to the relatively high median age of our cohort. The study design privileged autologous SCT over continuous combined therapy in good MRD responders without a donor. It is thus difficult to elaborate on how to select patients who could be treated without allogeneic SCT and which optimal treatment should be offered to such favorable patients. Nonetheless, our observations suggest that patients with a low WBC and/or those reaching a good early BCR-ABL1 MRD response could represent this group of favorable patients, as suggested by the study of Ravandi et al showing that achieving MMolR between 3 and 12 months posttreatment improved survival.27 Allogeneic SCT, however, appeared to be as effective in patients who did not reach early MMolR as in those who did.
We have recently reported that good early immunoglobulin/T-cell receptor (Ig/TCR) MRD response is the best tool to select patients with Ph-negative ALL who may not benefit from allogeneic SCT in CR1, surpassing conventional risk factors such as WBC.28,29 Here, WBC appeared to be more discriminant than early MRD response, and it is interesting to note that early MRD response was influenced not only by WBC but also by the bcr subtype. We found that patients with M-bcr had a lower MRD response rate than those with m-bcr, as previously suggested. However, in this study, we monitored BCR-ABL1 and not Ig/TCR MRD levels. These patients could need a longer time to reach a transcript-based BCR-ABL1 response, even if their DNA-based Ig/TCR response might have occurred earlier. Together, these observations support the concepts of more prolonged exposure to TKI prior to SCT and coupled BCR-ABL1 and Ig/TCR MRD monitoring in these patients, as planned in our next nilotinib GRAAPH trial.
Finally, it is of interest to comment on the role of autologous SCT. We observed a similar outcome in patients who reached early MMolR and received either allogeneic or autologous SCT. Of course, we are not claiming that autologous SCT is the best treatment option for good MRD responders. Nonetheless, these results suggest that nonallogeneic options could be preferred in favorable patients, as associated with less morbidity and short-term mortality. Continuing TKI/chemotherapy administration might yield similar results, as suggested by pediatric studies,23,24 although the outcome of the minority of negatively selected patients who did not receive any SCT was dismal in our study. In addition, patients receiving autologous SCT also received prophylactic posttransplant maintenance including TKI, which was not planned after allogeneic SCT. Recently, a small randomized trial comparing imatinib given either prophylactically or driven by MRD positivity after allogeneic SCT showed interesting results, with 5-year EFS and OS estimates at 83.9% and 80.1% vs 60.4% and 74.5%, in the prophylactic and MRD-triggered arms, respectively.30 These results suggest that there is a benefit in combining the immunologic activity of allogeneic SCT and TKIs to decrease the risk of relapse. Therefore, in the next GRAAPH trial, patients will also receive TKI maintenance after allogeneic SCT.
To conclude, this study (1) validates the interest of an initial TKI-based therapy in adults with Ph+ ALL; (2) confirms the role of allogeneic SCT in CR1 in these patients, especially in those with persistent MRD levels; and (3) strongly suggests that favorable patients with low WBC and/or those with good early MRD response could be treated with nonallogeneic postremission therapies, including, for instance, autologous SCT and long-term TKI maintenance. Nevertheless, the general outcome of Ph+ ALL remains unsatisfactory, and new strategies need to be found that combine new TKIs with less-intense chemotherapy and post-SCT maintenance and that integrate new therapeutic approaches such as bispecific T cell–engaging antibodies or chimeric antigen receptor T cells.31-34
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the patients and their families for participating in the trial; E. Boucher, F. Daniel, N. Klein, C. Mélot, F. Perry, and M. Sauvezie for their help in data monitoring; K. Desseaux for help with statistical analysis; N. Dedin and M.-C. Béné for reviewing the manuscript; N. Raus, I. Yakoub-Agha, and the Société Française de Greffe de Moelle et de Thérapie Cellulaire for sharing data; and Jean-Paul Vernant and André Delannoy for their longstanding intellectual support.
This study (#NCT00327678) was sponsored by the Paris Ile-de-France Regional Clinical Research Office and supported by grants from the Programme Hospitalier de Recherche Clinique (#AOM04144) in France, and the Swiss State Secretariat for Education, Research, and Innovation in Switzerland. Novartis Pharma provided imatinib. Amgen France provided a research grant.
Authorship
Contribution: All authors contributed to the study’s conception and design; V.L. provided administrative support; Y.C., X.T., F.H., E.R., T.L., P.R., S.L., M.E.-B., S.M., C.B., E.T., J.-F.L., N.I., and H.D. provided study materials or patients; Y.C., X.T., S.H., J.-M.C., C.A., M.L.-P., V.L., S.C., N.I., and H.D. collected and assembled the data; S.H., J.-M.C., and M.L.-P. provided a central review of molecular and cytogenetic data; Y.C., S.C., N.I., and H.D. analyzed and interpreted the data; Y.C., V.L., N.I., and H.D. wrote the manuscript; and all authors approved the final manuscript.
Conflict-of-interest disclosure: Y.C. and J.-M.C. have received honoraria for participation in symposia and advisory boards and funding for participation at meetings from Novartis. H.D. has received honoraria for participation in symposia and advisory boards and research funding from Novartis and Amgen. S.M. has received honoraria for participation in symposia from Amgen. J.-F.L. has received honoraria for participation in symposia and advisory boards and funding for participation at meetings from Novartis and Amgen. F.H. has received honoraria for participation in symposia and advisory boards from Novartis and Amgen. M.E.-B. has received honoraria for participation in advisory boards from Novartis. The remaining authors declare no competing financial interests.
This article is written on behalf of the GRAALL, which includes the former France-Belgium Group for Lymphoblastic Acute Leukemia in Adults, the French Western-Eastern Group for Lymphoblastic Acute Leukemia, and the Swiss Group for Clinical Cancer Research. The authors thank the GRAALL investigators for submitting clinical data and samples. A complete list of the members of the GRAALL appears in the online data supplement.
Correspondence: Yves Chalandon, Hemato-Oncology Unit, Hematology Division, Hôpitaux Universitaires de Genève, 4 rue Gabrielle Perret-Gentil, 1211 Geneva, Switzerland; e-mail: yves.chalandon@hcuge.ch.

![Figure 3. Role of SCT in CR1. Simon-Makuch plots for RFS (A) and OS (B) in CR patients. t0 was the time of hematologic CR achievement. RFS and OS were significantly prolonged in the allogeneic SCT cohort (HR, 0.69 [95% CI, 0.49-0.98; P = .036] and 0.64 [95% CI, 0.44-0.93; P = .020], respectively, by the Andersen-Gill test). Simon-Makuch plots for RFS (C) and OS (D) in patients in MMolR at MRD2 time point. t0 was the time of MRD2 MMolR achievement. RFS and OS were similar in the autologous and allogeneic SCT cohorts (HR, 0.94 [95% CI, 0.53-1.65; P = .82] and 0.95 [95% CI, 0.51-1.74; P = .86], respectively, by the Andersen-Gill test). A 3-month RFS landmark period (median time from CR to transplantation) was used, because patients should be alive but also in CR1 to be actually transplanted. This landmark allowed minimizing the bias related to early relapses when comparing OS with this method.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/24/10.1182_blood-2015-02-627935/4/m_3711f3.jpeg?Expires=1765898477&Signature=X4bkJJd-HwKAP9KCKtm4bDkw34BhRylKjH7g4tB~zj2aqxE56sF8u07wQKzMWhLG4Qv148znvl3LFMOsqYMH1tZHG4FQOh-a2J-LRhl3rb0iAmwnqo0NPSR1uL6R-fKFmvukDZ3Rl12tJahvQKL3EnK9LWz8mu9bLpEr9B6nNXecusLY2PqD4vyxSoGoIBpd2leRYacJzf~ZVyiN1pxdyTjlkPPdU5QiwvlX6D7zR6kUKHGgwUW-2YyHiumTbTJflPI1HEqtwk5rAR~MDj7th5HExV9L63P0BJWN3dYSzmVdF9hDBMvqHuFnxBxrj0ffDrWrH5NiGnA-~9UV~6wSsQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 3. Role of SCT in CR1. Simon-Makuch plots for RFS (A) and OS (B) in CR patients. t0 was the time of hematologic CR achievement. RFS and OS were significantly prolonged in the allogeneic SCT cohort (HR, 0.69 [95% CI, 0.49-0.98; P = .036] and 0.64 [95% CI, 0.44-0.93; P = .020], respectively, by the Andersen-Gill test). Simon-Makuch plots for RFS (C) and OS (D) in patients in MMolR at MRD2 time point. t0 was the time of MRD2 MMolR achievement. RFS and OS were similar in the autologous and allogeneic SCT cohorts (HR, 0.94 [95% CI, 0.53-1.65; P = .82] and 0.95 [95% CI, 0.51-1.74; P = .86], respectively, by the Andersen-Gill test). A 3-month RFS landmark period (median time from CR to transplantation) was used, because patients should be alive but also in CR1 to be actually transplanted. This landmark allowed minimizing the bias related to early relapses when comparing OS with this method.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/24/10.1182_blood-2015-02-627935/4/m_3711f3.jpeg?Expires=1766577392&Signature=4SaBoatz7Wf8rtIJYll1SGCsoF7Iru1R632OueNspgzhtjNI6NyA4ibFng2VSPPsjYoAljpSt2Ej4SC2Ba~d74IHSUsNe~rburi4NyvKWAcAUZ~4pjSp2gobbcDK2BI5Z6ODZaT5yBFNOtud~VKw3j4gWElxwBBg8seQiJfJsLtOPf0e2ck8QSM5061LQSJ3xYbNaTNcSPl~UQfNjAc8xp-Q~7OgkDPPWFQ266QLKrUAxY0WnsNoV1t-qXJ-uPrYtND0Kh7jVj5R1nqZwyomDudgDPCDbrDPCJaJC5w6LVNOoQ72U6xCaHc6yTNSE7R1vy2KOVZzzFu2~t1V~BJe~Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)