Key Points
Inactivation of either Srf or both Mrtfs in HSC/Ps prevents their colonization of the bone marrow.
HSC/Ps lacking functional Srf or both Mrtfs exhibit greatly reduced chemotactic response to SDF-1.
Abstract
Chemokine signaling is important for the seeding of different sites by hematopoietic stem cells (HSCs) during development. Serum response factor (SRF) controls multiple genes governing adhesion and migration, mainly by recruiting members of the myocardin-related transcription factor (MRTF) family of G-actin–regulated cofactors. We used vav-iCre to inactivate MRTF-SRF signaling early during hematopoietic development. In both Srf- and Mrtf-deleted animals, hematopoiesis in fetal liver and spleen is intact but does not become established in fetal bone marrow. Srf-null HSC progenitor cells (HSC/Ps) fail to effectively engraft in transplantation experiments, exhibiting normal proximal signaling responses to SDF-1, but reduced adhesiveness, F-actin assembly, and reduced motility. Srf-null HSC/Ps fail to polarize in response to SDF-1 and cannot migrate through restrictive membrane pores to SDF-1 or Scf in vitro. Mrtf-null HSC/Ps were also defective in chemotactic responses to SDF-1. Srf-null HSC/Ps exhibit substantial deficits in cytoskeletal gene expression. MRTF-SRF signaling is thus critical for expression of genes required for the response to chemokine signaling during hematopoietic development.
Introduction
Establishment and maintenance of the hematopoietic system involves coordinated control of the generation, renewal and differentiation of hematopoietic stem cells (HSCs), and their migration to and retention at specialized stem-cell niches. During development, HSCs are initially generated in the aorta/gonad/mesonephros (AGM) region, and possibly the yolk sac and placenta; they then move to the fetal liver before colonizing the bone marrow, just before birth.1,2 Homing and retention of HSCs involves a complex interplay among chemokine signaling, cytoskeletal dynamics, and extracellular matrix remodeling.3,4
The chemokines SDF-1 and SCF play particularly important roles in hematopoietic development and homeostasis.2,5 In mouse, inactivation of SDF-1 (Cxcl12) or its receptor (Cxcr4) greatly reduces HSC numbers in E18.5 fetal bone marrow but does not affect fetal liver HSC numbers,6-9 whereas postnatal inactivation of Cxcl12 mobilizes stem cells from the niche.10 Inactivation of the SCF(Kitl)-Kit axis has similar effects.5,11 Retention of HSCs in the endosteal niche, and presumably their establishment there, depends on production of SDF-1 and SCF in perivascular mesenchymal stromal cells.11-14
The serum response factor (SRF) transcription factor network couples cytoskeletal gene expression with extracellular signals and adhesive cues.15-17 SRF integrates proliferative and cytoskeletal signaling pathways through its interactions with 2 families of signal-regulated cofactors: the MAP-kinase–regulated ternary complex factors (TCFs: SAP-1, Elk1, and Net) and the G-actin–regulated myocardin-related transcription factors (MRTFs: MRTF-A and MRTF-B).16,17 In the hematopoietic system, TCF-SRF signaling is required for T-cell–positive selection and marginal zone B-cell formation,18-20 but fetal liver cells lacking all 3 TCFs can effectively reconstitute hematopoiesis.18 In contrast, MRTF-SRF signaling is required for megakaryocyte differentiation and platelet function.21 Functional Srf is also required for neutrophil migration and polarization22 ; its postnatal inactivation in adult hematopoietic cells mobilizes HSC/Ps23 and impairs macrophage adhesion, migration, and phagocytosis,24 but the SRF cofactors involved remain unknown.
Here we investigate MRTF-SRF signaling in early hematopoietic development. Inactivation of Srf in hematopoietic cells (SrfKOH animals) causes hemorrhage and perinatal death. Bone marrow from SrfKOH animals is hypocellular and virtually devoid of hematopoietic activity, although hematopoietic activity in the fetal liver and spleen is substantially unaffected, a phenotype reminiscent of defective SDF-1 or SCF signaling. SrfKOH fetal liver cells exhibit severely compromised engraftment and defective homing to bone marrow. Hematopoietic stem/progenitor cells (HSC/Ps) from SrfKOH animals exhibit adhesion defects and a significantly reduced motile response to SDF-1, and are severely compromised in their chemotactic response to SDF-1 and SCF. Animals lacking Mrtfa and Mrtfb also exhibit bone-marrow colonization failure and defective HSC/P chemotactic responses to SDF-1. MRTF-SRF signaling is thus required for chemokine responses during establishment of hematopoiesis in the developing embryo.
Methods
Mice
Animals were maintained under specific-pathogen–free conditions in the Cancer Research UK (CRUK) Biological Resources Unit. Animal experimentation, approved by the CRUK Animal Ethics committee, was carried out under Home Office license PPL 80/2602. For gene inactivation in hematopoietic cells, we used vav-iCre,25 with Srff/f,26 Mrtfa−/−, and Mrtfbfl/+.27 For some experiments, SRF network alleles were bred onto mT/mG28 or R26R-EYFP Cre reporter backgrounds, where reporter expression marks presumptive SrfKOH cells (supplemental Figure 3, available on the Blood Web site). For reconstitution, one week acid-watered C56BL6/SJL or NRG hosts were 137Cs-irradiated (C56BL6/SJL: 2 × 4.5 Gy or 2 × 6 Gy, 3-hour interval; NRG 1 × 5.5 Gy), and 24 hours later, fetal liver cells were injected into the tail vein. For homing, 1 × 105 fetal liver LSK cells (Srf+ and SrfKOH mT/mG, mixed 1:1) were injected into the tail vein of C57BL6 mice. For in vivo imaging,29 carboxyfluorescein succinimidyl ester (CFSE)- or SNARF-labeled LSK cells were injected into irradiated NRG mice (4.5 Gy).
HSC/P cell adhesion, morphology, and chemotactic migration assays
For adhesion assays, Srf+ (mT) and SrfKOH (MG) LSK cells (5 × 104 cells) were combined 1:1 and allowed to adhere to fibronectin-coated or MBA-2.1 endothelial cell substrates for 30 minutes before counting by fluorescence-activated cell sorting (FACS). For cell shape analysis, cells were plated on polycarbonate transwells, 100 ng/mL SDF-1 was added to the lower well for 45 minutes, and they were visualized by confocal microscopy. Circularity was measured using ImageJ; Z-stack analysis used custom software (see supplemental Methods). For transmigration assays 5 × 104 LSK cells (Srf+ and SrfKOH mixed at 1:1, Mrtf mutant cells by genotype) were plated polycarbonate transwells, with 100 ng/mL SDF-1 or SCF-1 in the bottom well, and migration analyzed by FACS. For motility assays, CFSE-labeled LSK cells were settled on MBA-2.1 monolayers, SDF-1 added, and cells tracked for 2 hours by time-lapse microscopy.
Other methods
Lineage-negative c-Kit+ Sca-1+ cells were purified on the BD FACS Aria III after disaggregation of livers from E14.5-15.5 embryos. For colony-forming unit (CFU) assays, cells were plated in Methocult (GF M34334, Stem Cell Technologies), and colonies were counted and scored as CFU-G, CFU-M, CFU-GM, and blast-forming unit erythroid (BFU-E) CFU-GEMM after 7 to 9 days of culturing. FACS analysis used the BD LSRII analyzer, with analysis using FlowJo 9.5.3 software. RNA-seq data are available under Gene Expression Omnibus accession number GSE63820.
Results
Srf is required to establish hematopoiesis in the bone marrow
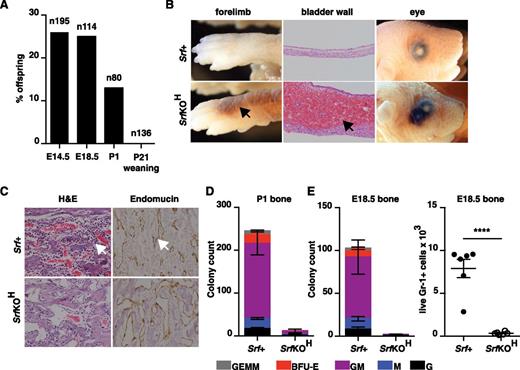
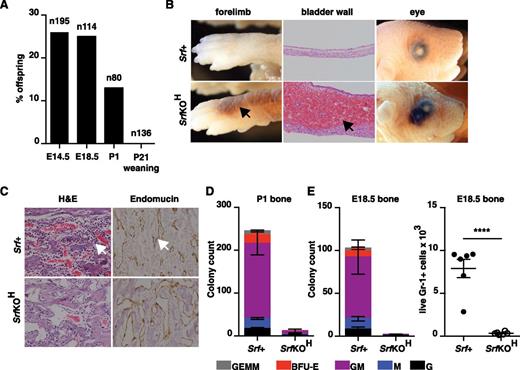
We used vav-iCre25 and the conditional Srf allele Srff/f 26 to inactivate Srf at the onset of hematopoiesis. No viable vav-iCre;Srff/f (referred to hereafter as SrfKOH) animals were obtained at 4 weeks, although normal Mendelian ratios were observed before birth (Figure 1A). Newborn pups died within 24 hours after failing to thrive and displaying extensive hemorrhage into the skin, lungs, and bladder, although no hemorrhage was observed in E14.5 and E18.5 embryos (Figure 1B; see “Discussion”). Histologic staining of bone sections from P1 SrfKOH animals revealed a paucity of mononuclear lymphoid cells, although the vasculature appeared intact (Figure 1C). Consistent with this, P1 or E18.5 SrfKOH bone marrow lacked colony-forming activity and was virtually devoid of live Gr-1+ cells (Figure 1D-E). In contrast, liver or spleen from SrfKOH E18.5 or P1 animals generated similar numbers of granulocyte (G), macrophage (M), granulocyte-monocyte (GM), BFU-E, and granulocyte-erythrocyte-macrophage (GEM) colonies (supplemental Figure 1A).
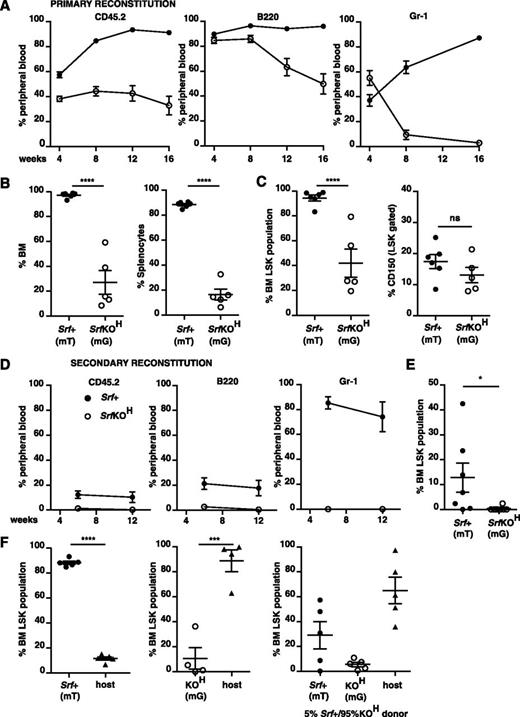
Early hematopoietic inactivation of Srf causes perinatal lethality and lack of bone marrow cellularity. (A) Embryos or animals were genotyped at the indicated stages and proportion of SrfKOH (vav-iCre; Srff/f) scored; n = total embryos/animals genotyped. (B) Hemorrhage into skin, bladder, or eye in newborn SrfKOH compared with Srf+ (Srff/f, Srff/+) animals. (C) P1 femurs stained with hematoxylin and eosin (H&E) (left) or endomucin (right). (D) Colony-formation assays with cells from the P1 femur of SrfKOH animals. Data are from 3 Srf+ and 3 SrfKOH animals, each assay was performed in triplicate. See supplemental Figure 1A. (E) Left, reduced colony-formation activity in hind-limb long bones of E18.5 SrfKOH animals. Data are from 5 Srf+ and 3 SrfKOH embryos; each assay was performed in triplicate. Right, Gr-1+ cellularity in E18.5 long bones. Data are from 6 embryos of each genotype (P < .0001; unpaired Student t test).
Early hematopoietic inactivation of Srf causes perinatal lethality and lack of bone marrow cellularity. (A) Embryos or animals were genotyped at the indicated stages and proportion of SrfKOH (vav-iCre; Srff/f) scored; n = total embryos/animals genotyped. (B) Hemorrhage into skin, bladder, or eye in newborn SrfKOH compared with Srf+ (Srff/f, Srff/+) animals. (C) P1 femurs stained with hematoxylin and eosin (H&E) (left) or endomucin (right). (D) Colony-formation assays with cells from the P1 femur of SrfKOH animals. Data are from 3 Srf+ and 3 SrfKOH animals, each assay was performed in triplicate. See supplemental Figure 1A. (E) Left, reduced colony-formation activity in hind-limb long bones of E18.5 SrfKOH animals. Data are from 5 Srf+ and 3 SrfKOH embryos; each assay was performed in triplicate. Right, Gr-1+ cellularity in E18.5 long bones. Data are from 6 embryos of each genotype (P < .0001; unpaired Student t test).
Srf is not essential for fetal liver hematopoiesis or fetal thymic seeding
To examine early stages of hematopoiesis, we analyzed embryonic fetal liver, in which polymerase chain reaction (PCR) analysis confirmed quantitative inactivation of Srf (supplemental Figure 1B). The cellularity of wild-type and SrfKOH E14.5 fetal livers was comparable (Figure 2A), but multipotent fetal liver HSC/P numbers (lin–Sca-1+c-kit+ “LSK” cells) increased approximately twofold in SrfKOH embryos, reflecting an increased proliferation rate (Figure 2B; see “Discussion”). The CD150+ LSK subpopulation, which is greatly enriched in HSCs,30 was present in comparable proportions in wild-type and SrfKOH fetal livers, indicating that Srf is not required for HSC generation per se (Figure 2C). Acute inactivation of Srf in adult bone marrow also increases LSK cell numbers23 (see “Discussion”). Wild-type and SrfKOH E14.5 fetal liver cells had equal hematopoietic colony-forming activity (Figure 2D). Although the SrfKOH colonies were markedly more compact than wild-type (supplemental Figure 1C), the total cell numbers were not significantly different, indicating that SrfKOH HSC/P proliferation was comparable with wild-type in this context (Figure 2E). Together with the bone marrow colonization defect, these hematopoietic phenotypes are strikingly similar to those resulting from inactivation of the Cxcr12/Cxcr46-9 or Kitl/Kit.5,11
Hematopoietic progenitor cells in SrfKOH fetal liver. (A) Cellularity of SrfKOH and Srf+ fetal liver. (B) Fetal liver LSK cells (see also supplemental Figure 1B). Panels Bi-ii, elevated numbers of LSK cells in SrfKOH fetal liver. Panel Biii, BrdU labeling indicates that SrfKOH LSK cells are more proliferative than those from Srf+ embryos. (C) Similar proportions of CD150hi cells in SrfKOH fetal liver. (D) SrfKOH and Srf+ fetal liver cells generate similar numbers of colonies in colony-formation assays. Data are from 6 Srf+ and 4 SrfKOH embryos; each assay was performed in triplicate. See supplemental Figure 1C. (E) Although SrfKOH and Srf+ colony morphologies are different (i), the total cell numbers are similar (ii).
Hematopoietic progenitor cells in SrfKOH fetal liver. (A) Cellularity of SrfKOH and Srf+ fetal liver. (B) Fetal liver LSK cells (see also supplemental Figure 1B). Panels Bi-ii, elevated numbers of LSK cells in SrfKOH fetal liver. Panel Biii, BrdU labeling indicates that SrfKOH LSK cells are more proliferative than those from Srf+ embryos. (C) Similar proportions of CD150hi cells in SrfKOH fetal liver. (D) SrfKOH and Srf+ fetal liver cells generate similar numbers of colonies in colony-formation assays. Data are from 6 Srf+ and 4 SrfKOH embryos; each assay was performed in triplicate. See supplemental Figure 1C. (E) Although SrfKOH and Srf+ colony morphologies are different (i), the total cell numbers are similar (ii).
Inactivation of Srf in late thymopoiesis blocks thymocyte positive selection.19,20 Thymic cellularity of E17.5 SrfKOH embryos was comparable with wild-type, as were double-positive (DP) (CD4+ CD8+) thymocyte numbers, indicating that SRF is not required before positive selection (supplemental Figure 2A) and, as expected, SrfKOH E15.5 thymus generated DP but not single-positive thymocytes during 5 days of foetal thymic organ culture, FTOC (see “Discussion”; supplemental Figure 2B). SrfKOH embryonic thymuses contained significantly reduced numbers of lineage-negative CD44+ CD25– CD117+ early thymocyte progenitors (ETPs), although their cellularity was normal, presumably owing to compensatory expansion of more mature thymocyte populations (supplemental Figure 2C; discussed later).
Srf is required for durable bone marrow engraftment
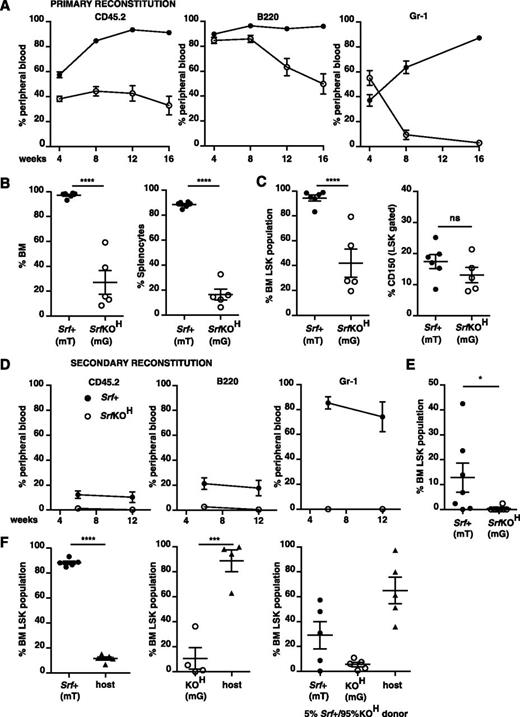
To investigate the ability of SrfKOH cells to colonize hematopoietic niches, we analyzed bone marrow engraftment by fetal liver LSK cells. To detect donor cells we used either the CD45.2 marker or we tracked Srf inactivation status by using the mT/mG reporter system,28 whereby membrane-Tomato or membrane-GFP expression identifies Srf+ (Cre-inactive) and SrfKOH (Cre-active) cells, respectively (supplemental Figure 3).
Despite the elevated numbers of LSK cells in SrfKOH fetal liver, reconstitution was only effective after high-dose irradiation, and even then, B220+ lineages declined steadily from a high initial contribution, whereas Gr-1+ lineages declined precipitately (Figure 3A and supplemental Figure 4A-B). Both spleen and bone marrow exhibited defective SrfKOH donor contribution at 18 weeks (Figure 3B); SrfKOH LSK numbers were correspondingly substantially reduced, although the relative proportion of CD150hi cells in the SrfKOH and wild-type LSK populations were similar (Figure 3C). Consistent with this, sorted SrfKOH bone marrow from animals reconstituted with SrfKOH fetal liver cells failed in a secondary reconstitution experiment: although recipient animals survived, SrfKOH cells in peripheral blood declined below detection limits 12 weeks postreconstitution (Figure 3D and supplemental Figure 4C), as did numbers of SrfKOH LSK cells in the reconstituted bone marrow (Figure 3E).
SrfKOH fetal liver cells fail to engraft durably. 106 total CD45.2+Srf+ or SrfKOH fetal liver cells were used to reconstitute wild-type CD45.1+ animals after high-dose (2 × 6 Gy) irradiation as indicated. Donor Srf+ and SrfKOH cells were distinguished by CD45.2 marker or use of the mT/mG system (mT, Srf+; mG, SrfKOH; see supplemental Figure 3). Solid symbols, Srf+; open symbols, SrfKOH. For raw data and results after low-dose irradiation (2 × 4.5 Gy), see supplemental Figure 4. (A) Peripheral blood analysis. Left, total donor and host cells were distinguished using CD45.2 (donor) and CD45.1 (host); middle and right, donor cells were analyzed for contribution of Srf+ (mT) or SrfKOH (mG) cells to B220 (B cell) or Gr-1 (granulocyte) lineages by gating on mT or mG as appropriate. Mean values are shown (n = 4-6 per condition). (B) Srf+ (mT) and SrfKOH (mG) cells in reconstituted bone marrow (left) and spleen (right) at 18 weeks. (C) Srf+ (mT) and SrfKOH (mG) LSK cells in reconstituted bone marrow at 18 weeks. Left, LSK-gated bone marrow; right, proportion of CD150+ cells. (D) Secondary transplants of bone marrow cells from animals reconstituted for 18 weeks with Srf+ or SrfKOH fetal liver cells. Reconstitutions were performed using 2 × 105Srf+ (mT) or SrfKOH (mG) bone marrow cells; irradiation was at 2 × 6 Gy. Analysis was done as in (A). See also supplemental Figure 4C. (E) Proportion of LSK cells in bone marrow 16 weeks after the secondary transplant in (C). (F) SrfKOH cells compete ineffectively with Srf+ cells for bone marrow engraftment. Donor Srf+ and SrfKOH cells were engrafted either alone (left and center panels) or in 1:19 Srf+:SrfKOH ratio (right panel) after 2 × 6 Gy irradiation, and the proportions of LSK-gated Srf+ (mT), SrfKOH (mG), and host cells were measured 12 weeks later.
SrfKOH fetal liver cells fail to engraft durably. 106 total CD45.2+Srf+ or SrfKOH fetal liver cells were used to reconstitute wild-type CD45.1+ animals after high-dose (2 × 6 Gy) irradiation as indicated. Donor Srf+ and SrfKOH cells were distinguished by CD45.2 marker or use of the mT/mG system (mT, Srf+; mG, SrfKOH; see supplemental Figure 3). Solid symbols, Srf+; open symbols, SrfKOH. For raw data and results after low-dose irradiation (2 × 4.5 Gy), see supplemental Figure 4. (A) Peripheral blood analysis. Left, total donor and host cells were distinguished using CD45.2 (donor) and CD45.1 (host); middle and right, donor cells were analyzed for contribution of Srf+ (mT) or SrfKOH (mG) cells to B220 (B cell) or Gr-1 (granulocyte) lineages by gating on mT or mG as appropriate. Mean values are shown (n = 4-6 per condition). (B) Srf+ (mT) and SrfKOH (mG) cells in reconstituted bone marrow (left) and spleen (right) at 18 weeks. (C) Srf+ (mT) and SrfKOH (mG) LSK cells in reconstituted bone marrow at 18 weeks. Left, LSK-gated bone marrow; right, proportion of CD150+ cells. (D) Secondary transplants of bone marrow cells from animals reconstituted for 18 weeks with Srf+ or SrfKOH fetal liver cells. Reconstitutions were performed using 2 × 105Srf+ (mT) or SrfKOH (mG) bone marrow cells; irradiation was at 2 × 6 Gy. Analysis was done as in (A). See also supplemental Figure 4C. (E) Proportion of LSK cells in bone marrow 16 weeks after the secondary transplant in (C). (F) SrfKOH cells compete ineffectively with Srf+ cells for bone marrow engraftment. Donor Srf+ and SrfKOH cells were engrafted either alone (left and center panels) or in 1:19 Srf+:SrfKOH ratio (right panel) after 2 × 6 Gy irradiation, and the proportions of LSK-gated Srf+ (mT), SrfKOH (mG), and host cells were measured 12 weeks later.
These results show that SrfKOH fetal liver contains cells that can repopulate the hematopoietic system, but that engraftment is not durable. To test whether SrfKOH HSC/Ps compete inefficiently with radio-resistant host stem cells for homing and/or retention at the niche, we performed competitive reconstitution using Srf+ and SrfKOH fetal liver cells in a 1:19 ratio. At 12 weeks postreconstitution, only ∼35% of LSK cells were donor-derived, with Srf+ and SrfKOH present at a 6:1 ratio, the remaining 65% of LSK cells being host-derived (Figure 3F). Taken together, these results show that although SrfKOH cells can support short-term engraftment, long-term engraftment is ineffective, at least in part owing to their inability to compete with wild-type cells.
Srf is required for effective thymic reconstitution
Maintenance of the postnatal thymus depends on continuous replenishment by progenitors originating in bone marrow,31,32 and thymic reconstitution thus depends on effective bone marrow engraftment. Fetal liver cells lacking all 3 TCFs can efficiently reconstitute the thymus, even at low irradiation dose.18 Thymic reconstitution by SrfKOH fetal liver cells was ineffective even after lethal irradiation, being proportionate to the fraction of donor LSK cells in the reconstituted bone marrow (supplemental Figure 5A). In contrast, in NOD/RAG/γC (NRG) hosts, whose thymic lobes are virtually devoid of competing cells, SrfKOH fetal liver could generate thymuses, albeit with delayed kinetics (supplemental Figure 5B). As seen in SrfKOH embryonic thymus, thymocyte development in these reconstituted thymuses did not progress beyond the DP stage (supplemental Figure 5C), and the proportions of double-negative thymocytes and ETPs were greatly reduced (supplemental Figure 5D).
Fetal HSC/P homing requires Srf
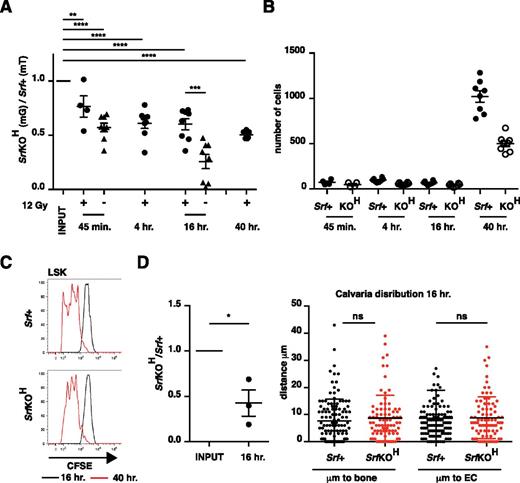
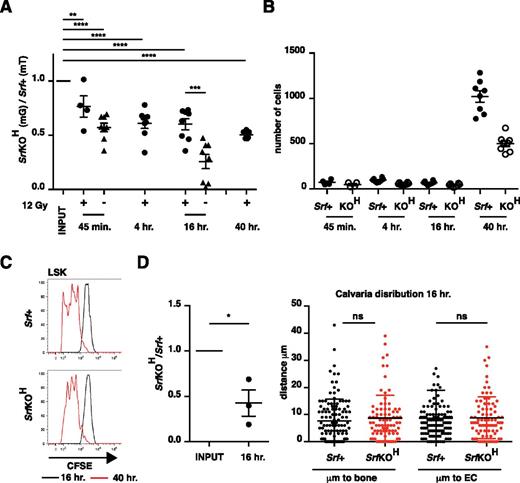
Although the ineffective competition between SrfKOH and Srf+ cells can explain their defective engraftment properties, it cannot explain the failure of SrfKOH cells to colonize the bone marrow during development because competing wild-type cells are absent, so we investigated other aspects of LSK homing. In the transplanted adult mouse, HSCs rapidly disappear from circulation, arriving at their target hematopoietic niches within minutes.33 Equal numbers of Srf+ and SrfKOH fetal liver LSK cells were mixed and analyzed in short-term homing assays.34 Even 45 minutes postinjection SrfKOH LSK cells exhibited 50% reduced accumulation in long bone; this deficit was more marked in nonirradiated animals, suggesting that it can be partially suppressed by increased chemokine signaling or vascular permeability (Figure 4A). After arrival at the bone, numbers remained constant for up to 16 hours, after which both SrfKOH and Srf+ LSK cells underwent similar proliferative expansion, increasing tenfold by 40 hours (Figure 4B). CFSE staining experiments showed that SrfKOH and Srf+ LSK cells proliferated comparably (Figure 4C).
Homing to bone is defective in SrfKOH cells. (A) Srf+ and SrfKOH fetal liver LSK cells were mixed 1:1 and 105 cells injected into the tail vein of C57Bl6 mice, with prior irradiation where indicated. The ratio of SrfKOH to Srf+ cells present in the hind-limb long bones was evaluated at the indicated times. (B) Absolute numbers of cells homed to the long bones at different times after injection in the experiment shown in (A). (C) Proliferation of CFSE-labeled cells homed to long bones at different times after injection. (D) Homing to calvaria. Animals were injected with fetal liver LSK cells from Srf+ and SrfKOH animals, labeled with CFSE and SNARF, respectively, to increase detection sensitivity, mixed 1:1. Left, relative proportions of Srf+ and SrfKOH cells in calvaria 16 hours after injection. Right, distance of each homed LSK cell from the nearest bone or endothelial cell. Error bars = standard error of the mean.
Homing to bone is defective in SrfKOH cells. (A) Srf+ and SrfKOH fetal liver LSK cells were mixed 1:1 and 105 cells injected into the tail vein of C57Bl6 mice, with prior irradiation where indicated. The ratio of SrfKOH to Srf+ cells present in the hind-limb long bones was evaluated at the indicated times. (B) Absolute numbers of cells homed to the long bones at different times after injection in the experiment shown in (A). (C) Proliferation of CFSE-labeled cells homed to long bones at different times after injection. (D) Homing to calvaria. Animals were injected with fetal liver LSK cells from Srf+ and SrfKOH animals, labeled with CFSE and SNARF, respectively, to increase detection sensitivity, mixed 1:1. Left, relative proportions of Srf+ and SrfKOH cells in calvaria 16 hours after injection. Right, distance of each homed LSK cell from the nearest bone or endothelial cell. Error bars = standard error of the mean.
In bone marrow, HSCs become established close to blood vessels.2 To examine the destination of homed fetal liver LSK cells more closely, we imaged calvaria 16 hours postinjection using mixed populations of SrfKOH and Srf+ LSK cells. As seen with the long bones, the numbers of SrfKOH cells present in the calvaria were approximately halved compared with Srf+ cells, but there was no significant difference in their location, which was predominantly within 10 µm of endothelial cells (Figure 4D).
SrfKOH HSC/P cells exhibit defective adhesion and fail to polarize or migrate in response to SDF-1
The SrfKOH bone colonization phenotype is strikingly similar to that seen upon inactivation of SDF-1 signaling.6-9 Because SDF-1 signaling stimulates migration, adhesion, and transendothelial migration by HSC/Ps,3 we investigated SDF-1 signaling in SrfKOH animals. Expression levels of Cxcr4 on the surface of Srf+ and SrfKOH LSK cells were comparable (supplemental Figure 6A), and SDF-1 stimulation of purified SrfKOH LSK cells activated ERK and PI3 kinase signaling indistinguishably from Srf+ cells (supplemental Figure 6B). Levels of F-actin were somewhat reduced in SrfKOH LSK cells, but increased similarly to Srf+ cells upon SDF-1 stimulation (supplemental Figure 6C). Thus, at least the proximal SDF-1 signaling events do not require Srf.
Srf inactivation impairs cell adhesion in several systems.17 SrfKOH fetal liver LSK cells exhibited significantly reduced cell-surface expression of integrins β1 (∼30%), β2 (∼10%), and α4 (∼15%), components of the major hematopoietic adhesion complexes VLA-4, VLA-5, and LFA-1, although no changes in expression were observed in RNA-seq and quantitative real-time PCR analysis (supplemental Figure 6D-E); inactivation of Srf in adult bone marrow LSKs has a similar effect.23 Consistent with this, SrfKOH fetal liver LSK cells were less adherent, both to fibronectin (FN) and to MBA-2.1 endothelial cell monolayers, although adhesion to both substrates was increased in the presence of 100 ng/mL SDF-1 (Figure 5A). SDF-1 induced polarization of LSK cells plated on FN-coated transwells, which was slightly reduced in SrfKOH cells, and a more marked polarization was induced in LSKs plated on MBA-2.1 monolayers, which was effectively abolished in SrfKOH cells (Figure 5B and supplemental Figure 7A). Polarization was associated with the flattening of LSK cells and their movement into the endothelial monolayer toward the source of SDF-1, whereas SrfKOH cells remained on the surface of the monolayer (Figure 5C). Upon plating on MBA-2.1 cells in the presence of SDF-1, SrfKOH LSK cells moved 40% more slowly but showed no change in persistence (Figure 5D).
SrfKOH HSC/P cells exhibit defective adhesion, polarization, and motile responses to SDF-1. (A) Adhesion analysis. Srf+ and SrfKOH fetal liver LSK cells were mixed 1:1 and seeded on fibronectin-coated substrate (i) or on monolayers of MBA-2.1 endothelial cells (ii), in the presence or absence of 100 ng/mL SDF-1. Solid symbols, Srf+; open symbols, SrfKOH. See supplemental Figure 6D. (B) Morphology analysis. Cells were plated as in (A) on fibronectin-coated or MBA-2.1 endothelial cell monolayers, allowed to settle, and then stimulated for 45 minutes with SDF-1 before fixing and visualization with either Texas Red phalloidin (FN) or by prestaining with CFSE (MBA-2.1). Cell shape, defined as circularity = 4π (area/perimeter2 ) was measured. A perfect circle has circularity 1.0, which decrease with increasing elongation. Solid symbols, Srf+; open symbols, SrfKOH. See supplemental Figure 7A. (C) Transendothelial migration. CFSE-stained LSK cells were plated on SNARF-stained MBA-2.1 monolayers and treated as in (B). Panel Ci, cell locations were displayed by cumulative CFSE pixel intensity (LSKs, black lines) and [1-cumulative pixel intensity] (MBA-2.1, red line) relative to the membrane surface. Panel Cii, representative images: LSK cells in green and MBA-2.1 cells in semitransparent red. (D) Cell migration. LSK cells were plated on MBA2.1 cells and allowed to adhere for 20 minutes, and SDF-1 was added, followed by tracking for 20 minutes. The rose plots map the movement of each cell during the course of the experiment relative to its position at time zero. Data from 243 Srf+ and 228 SrfKOH LSK cells tracked in 3 independent experiments are summarized.
SrfKOH HSC/P cells exhibit defective adhesion, polarization, and motile responses to SDF-1. (A) Adhesion analysis. Srf+ and SrfKOH fetal liver LSK cells were mixed 1:1 and seeded on fibronectin-coated substrate (i) or on monolayers of MBA-2.1 endothelial cells (ii), in the presence or absence of 100 ng/mL SDF-1. Solid symbols, Srf+; open symbols, SrfKOH. See supplemental Figure 6D. (B) Morphology analysis. Cells were plated as in (A) on fibronectin-coated or MBA-2.1 endothelial cell monolayers, allowed to settle, and then stimulated for 45 minutes with SDF-1 before fixing and visualization with either Texas Red phalloidin (FN) or by prestaining with CFSE (MBA-2.1). Cell shape, defined as circularity = 4π (area/perimeter2 ) was measured. A perfect circle has circularity 1.0, which decrease with increasing elongation. Solid symbols, Srf+; open symbols, SrfKOH. See supplemental Figure 7A. (C) Transendothelial migration. CFSE-stained LSK cells were plated on SNARF-stained MBA-2.1 monolayers and treated as in (B). Panel Ci, cell locations were displayed by cumulative CFSE pixel intensity (LSKs, black lines) and [1-cumulative pixel intensity] (MBA-2.1, red line) relative to the membrane surface. Panel Cii, representative images: LSK cells in green and MBA-2.1 cells in semitransparent red. (D) Cell migration. LSK cells were plated on MBA2.1 cells and allowed to adhere for 20 minutes, and SDF-1 was added, followed by tracking for 20 minutes. The rose plots map the movement of each cell during the course of the experiment relative to its position at time zero. Data from 243 Srf+ and 228 SrfKOH LSK cells tracked in 3 independent experiments are summarized.
Srf is required for the chemotactic response of HSC/Ps
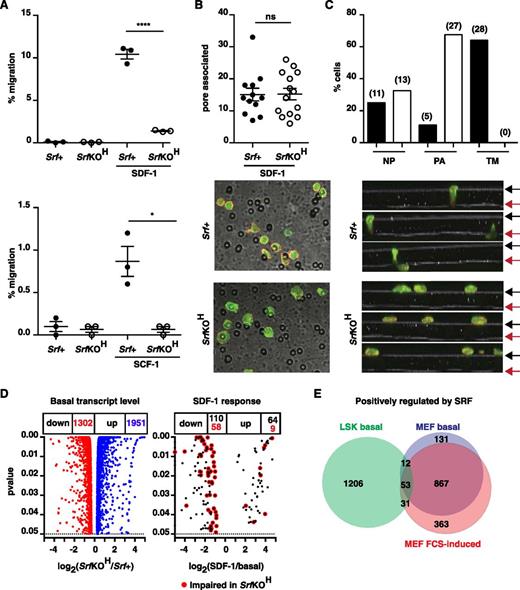
We investigated the chemotactic response of SrfKOH fetal liver LSK cells using a transwell assay. Compared with Srf+ cells, SrfKOH cells exhibited an 80% to 90% reduction in transit through a 5-µM pore toward SDF-1, and similar results were obtained using SCF; in both cases, transmigration was chemokine-dependent (Figure 6A). Transmigration through an 8-µM pore was more efficient but less Srf-dependent (supplemental Figure 7B). We did not observe cooperativity between SDF-1 and SCF, although this was previously reported.35 Despite the lower motility of SrfKOH LSK cells, they accumulated in numbers comparable with Srf+ cells at the membrane pores at early times during the experiment (Figure 6B). However, although Srf+ LSK cells could be readily visualized at pore entrances and transiting the pore at early times, SrfKOH FL LSK cells remained trapped at the pore entrance (Figure 6C).
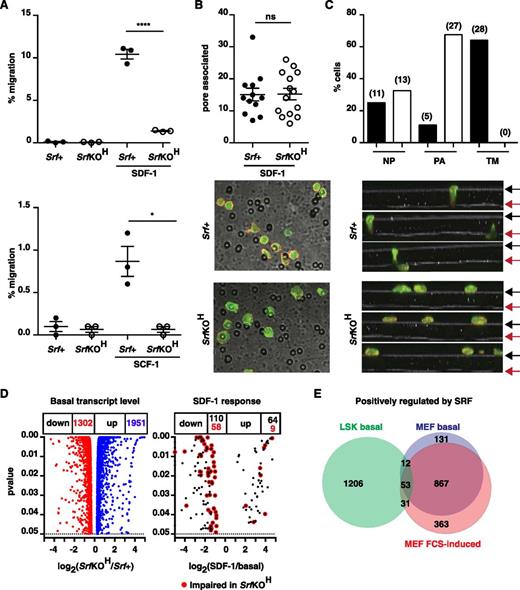
Srf and the Mrtfs are required for the chemotactic response to SDF-1. (A) Srf+ (mT) and SrfKOH (mG) fetal liver LSK cells were mixed 1:1, plated onto fibronectin-coated 5-µM pore transwells, and allowed to migrate across the membrane toward SDF-1 or SCF for 4 hours. See supplemental Figure 7B. (B) Srf+ or SrfKOH fetal liver LSK cells prelabeled with CFSE were plated onto the upper chamber of a fibronectin-coated 5-µM pore transwell and allowed to migrate toward SDF-1 for 45 minutes. After fixation, cells were stained with Texas Red phalloidin and imaged by confocal microscopy, and the cells associated with pores in 12 to 13 fields were counted. (C) Srf+ or SrfKOH fetal liver LSK cells were plated and processed as in (B), and Z-stacks acquired. Top panels, proportions of cells at different locations. Black and red arrows, upper and lower membrane surfaces. Bottom, cell numbers: NP, not associated with pores; PA, pore-entrance–associated; TM, transiting pore. (D) RNA-seq analysis of fetal liver LSK cells. Volcano plots of fold-change in RNA expression upon Srf inactivation vs statistical significance (left) and of SDF-1 induced transcripts in Srf+ cells. (E) Comparison of positively-regulated Srf-dependent gene sets in LSK and MEFs at significance P < .05. When a fold-change threshold of 2 is set for Srf dependence, 245 genes are positively regulated in LSKs, 657 in MEFs, and only 12 in both cell types. 1951 genes were negatively regulated by Srf in LSKs, of which 155 were shared with MEFs.
Srf and the Mrtfs are required for the chemotactic response to SDF-1. (A) Srf+ (mT) and SrfKOH (mG) fetal liver LSK cells were mixed 1:1, plated onto fibronectin-coated 5-µM pore transwells, and allowed to migrate across the membrane toward SDF-1 or SCF for 4 hours. See supplemental Figure 7B. (B) Srf+ or SrfKOH fetal liver LSK cells prelabeled with CFSE were plated onto the upper chamber of a fibronectin-coated 5-µM pore transwell and allowed to migrate toward SDF-1 for 45 minutes. After fixation, cells were stained with Texas Red phalloidin and imaged by confocal microscopy, and the cells associated with pores in 12 to 13 fields were counted. (C) Srf+ or SrfKOH fetal liver LSK cells were plated and processed as in (B), and Z-stacks acquired. Top panels, proportions of cells at different locations. Black and red arrows, upper and lower membrane surfaces. Bottom, cell numbers: NP, not associated with pores; PA, pore-entrance–associated; TM, transiting pore. (D) RNA-seq analysis of fetal liver LSK cells. Volcano plots of fold-change in RNA expression upon Srf inactivation vs statistical significance (left) and of SDF-1 induced transcripts in Srf+ cells. (E) Comparison of positively-regulated Srf-dependent gene sets in LSK and MEFs at significance P < .05. When a fold-change threshold of 2 is set for Srf dependence, 245 genes are positively regulated in LSKs, 657 in MEFs, and only 12 in both cell types. 1951 genes were negatively regulated by Srf in LSKs, of which 155 were shared with MEFs.
Multiple cytoskeletal genes are Srf-dependent in LSKs
We used RNA-seq to analyze LSK transcription. Srf inactivation significantly affected ∼18.5% of the 17 538 genes expressed in E14.5 LSK cells (1302 down, 1951 up; P < .05), and SDF-1 stimulation upregulated 64 transcripts within 30 minutes, of which 9 were Srf-dependent (Figure 6D and supplemental Tables 1 and 2). Genes positively regulated by Srf in LSKs are heavily enriched in genes involved in GTPase signaling, cell adhesion, and F-actin–based processes (supplemental Table 3). A similar analysis of MEFs revealed that ∼20.3% of 14 428 expressed genes were Srf-dependent (1457 down, 1475 up), with genes positively regulated by Srf also enriched in cytoskeletal categories (supplemental Table 3). However, only 96 Srf-dependent positively-regulated genes were shared between HSC/Ps and MEFs (Figure 6E). Thus, although Srf is a general regulator of cytoskeletal gene expression, its targets are cell context–dependent (see “Discussion”).
Bone marrow colonization requires Mrtfa and Mrtfb
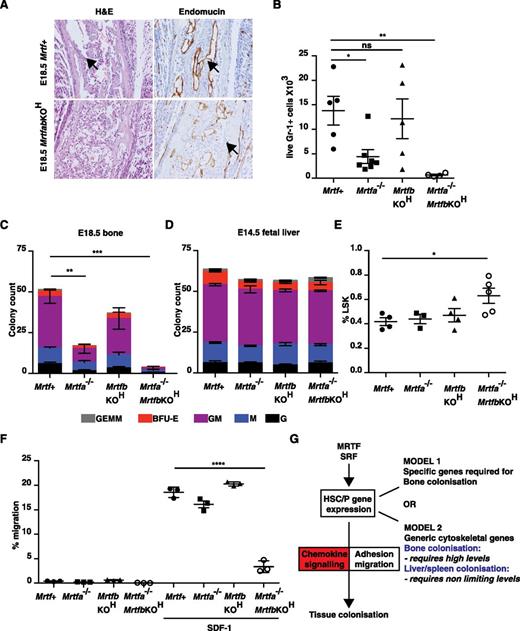
Cells lacking all 3 TCFs can reconstitute hematopoiesis,18 suggesting that the SrfKOH phenotype here reflects defective MRTF-SRF signaling. To test this idea, we combined viable Mrtfa null and conditional Mrtfb (Mrtfbf/f) alleles27 with vav-iCre. No vav-iCre;Mrtfa−/−;Mrtfbf/f (referred to hereafter as MrtfabKOH) animals were obtained, although E18.5 MrtfabKOH embryos were present in expected Mendelian ratios. However, vav-iCre;Mrtfa−/−;Mrtfbf/+ and vav-iCre;Mrtfa−/+;Mrtfbf/f animals were readily obtained, suggesting that a single copy of either Mrtfa or Mrtfb in hematopoietic cells suffices for viability (see “Discussion”).
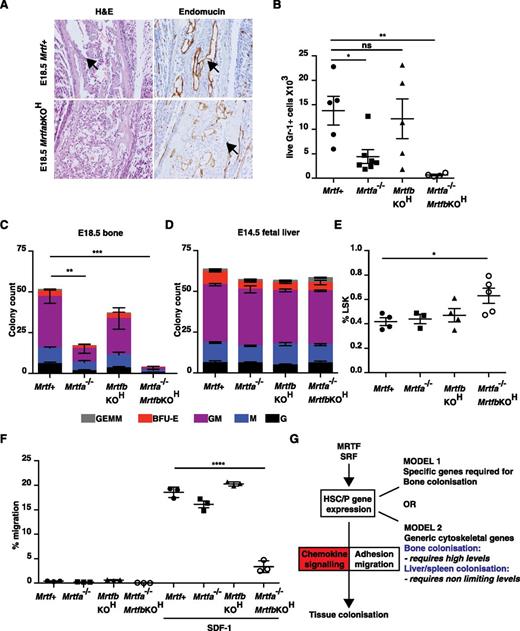
As with the SrfKOH embryos, examination of bone sections from E18.5 MrtfabKOH animals revealed an absence of mononuclear lymphoid cells, although the vasculature appeared intact (Figure 7A). Bone from E18.5 MrtfabKOH embryos lacked Gr-1+ cells and contained virtually no colony-forming activity; significantly reduced numbers of Gr-1+ cells and CFU activity were also seen in E18.5 Mrtfa−/− embryonic bone, although vav-iCre;Mrtfbf/f (referred to hereafter as MrtfbKOH) mice had only a mild defect (Figure 7B-C). Although numbers of LSK cells in fetal liver were only increased in MrtfabKOH embryos (Figure 7D), as with Srf, the colony-forming activity of fetal liver cells was similar regardless of Mrtf genotype (Figure 7E), although the colonies generated by MrtfabKOH fetal liver cells exhibited a compact morphology similar to those of SrfKOH colonies (supplemental Figure 8A). MrtfabKOH fetal liver cells also exhibited defective host engraftment, contributing poorly to the B220+ and Gr-1+ lineages, even at early times (supplemental Figure 8B).
Inactivation of both Mrtfs phenocopies SRF deletion. (A) E18.5 femurs stained with H&E (left) or endomucin (right). (B) Gr-1+ cellularity in mutant Mrtf mutant E18.5 long bones. Data are from 6 embryos of each genotype (P < .0001; unpaired Studen t test). (C) Colony-formation assays by cells from Mrtf-mutant MrtfabKOH E18.5 long bones. Data are from 3 embryos of each genotype, and each assay was performed in triplicate. (D) Proportion of LSK cells in Mrtf E14.5–mutant fetal livers. (E) Colony-formation assays with cells from Mrtf-mutant E14.5 fetal livers. Data are from 3 embryos of each genotype, and each assay was performed in triplicate. (F) Mrtf+, Mrtfa−/−, MrtfbKOH, or MrtfabKOH fetal liver LSK cells were mixed and plated onto fibronectin-coated 5-µM pore transwells and allowed to migrate across the membrane toward SDF-1 for 4 hours. (G) Roles of MRTF-SRF and chemokine signaling in tissue colonization by HSC/P. See “Discussion” for details.
Inactivation of both Mrtfs phenocopies SRF deletion. (A) E18.5 femurs stained with H&E (left) or endomucin (right). (B) Gr-1+ cellularity in mutant Mrtf mutant E18.5 long bones. Data are from 6 embryos of each genotype (P < .0001; unpaired Studen t test). (C) Colony-formation assays by cells from Mrtf-mutant MrtfabKOH E18.5 long bones. Data are from 3 embryos of each genotype, and each assay was performed in triplicate. (D) Proportion of LSK cells in Mrtf E14.5–mutant fetal livers. (E) Colony-formation assays with cells from Mrtf-mutant E14.5 fetal livers. Data are from 3 embryos of each genotype, and each assay was performed in triplicate. (F) Mrtf+, Mrtfa−/−, MrtfbKOH, or MrtfabKOH fetal liver LSK cells were mixed and plated onto fibronectin-coated 5-µM pore transwells and allowed to migrate across the membrane toward SDF-1 for 4 hours. (G) Roles of MRTF-SRF and chemokine signaling in tissue colonization by HSC/P. See “Discussion” for details.
Finally, we used the transwell assay to examine the chemotactic response of Mrtf-mutant LSK cells. MrtfabKOH cells also exhibited substantially defective transwell migration in response to SDF-1; inactivation of Mrtfa or Mrtfb individually had no significant effect, although a slight impairment of transwell migration was observed in animals both lacking Mrtfa and retaining a single functional Mrtfb allele (Figure 7F and supplemental Figure 8C). These results suggest that the defects in bone colonization in vivo and response to SDF-1 in vitro seen in SrfKOH animals arise from defective MRTF-SRF signaling, and that the 2 Mrtfs function redundantly in this setting.
Discussion
We investigated the role of the SRF network in hematopoietic development by targeted inactivation of Srf or its cofactors Mrtfa and Mrtfb. Our results indicate that MRTF-SRF signaling plays an essential role in establishment of definitive hematopoiesis in bone. Animals lacking functional Srf or both Mrtfs in the hematopoietic system exhibit a >95% reduction in perinatal bone marrow cellularity with a corresponding decrease in myeloerythroid colony-forming activity. Fetal liver HSC/Ps lacking functional Srf fail to engraft bone marrow efficiently and durably, and display a greatly reduced ability to reconstitute the thymus. SrfKOH HSC/Ps are defective in homing to the bone marrow, exhibit substantially decreased adhesion and motility, and both they and MrtfabKOH HSC/Ps also exhibit a severely impaired migratory response to SDF-1. SrfKOH HSC/Ps exhibit substantial deficits in cytoskeletal gene expression. Thus, MRTF-SRF signaling is essential for expression of genes required for the response to chemokines during hematopoietic development (Figure 7G).
We found that Srf inactivation in hematopoietic cells does not affect the generation of HSC and colony-forming activity in the fetal liver but instead prevents the colonization of the bone marrow during development. This phenotype is strikingly similar to that seen when the SCF-Kit5,11 or SDF-1/Cxcl12-Cxcr46-9 pathways are inactivated. Production of SCF/Kitl and SDF-1/Cxcl12 by bone-marrow stromal cells is required for establishment and retention of HSC in bone.10-14 We also found increased numbers and increased proliferation of SrfKOH HSC/P in embryonic liver: this probably also reflects a defective SDF-1 response, because increased stem-cell mobilization and proliferation of adult bone marrow HSC/P is induced upon Srf 23 or Cxcl1210 inactivation. We note that the total Cxcl12 or Kitl knockout mutations result in multiple other phenotypes not phenocopied by Srf deletion, which presumably reflect Srf-independent processes. For example, although SDF-1 signaling is required for early B-cell development, Srf is not.19
Our findings suggest that the requirement for MRTF-SRF signaling in bone marrow colonization reflects its involvement in the chemotactic and adhesive responses to SDF-1 and SCF. SDF-1 induces activation of adhesion molecules, including the β1 and β2 integrin dimers LFA-1, VLA4, and VLA5, allowing initial tethering of stem cells to the endothelial cell surface, after which they migrate across the endothelial layer to seed the endosteal niche.3 We found that SrfKOH HSC/Ps exhibited reduced expression of α2, β1, and β2 integrins, in agreement with a previous study of Srf-null adult bone marrow HSC/Ps,23 although this control appeared posttranscriptional in our cells and reduced adhesiveness both to the extracellular matrix and endothelial cells. Inactivation of Srf also impaired polarization, F-actin assembly, and motile responses induced by SDF-1 in HSC/Ps, even though proximal SDF-1 signaling remained intact. SrfKOH HSC/Ps also exhibited markedly defective chemotactic responses to SDF-1 and SCF, with transit through confined spaces and across endothelial cell sheets becoming rate limiting. Gene expression analysis revealed that inactivation of Srf reduced the expression of dozens of genes involved in the structure and regulation of cytoskeleton in HSC/Ps, and that only very few of these are induced by SDF-1. We thus favor the view that the failure to establish effective hematopoiesis in the bone marrow during development reflects a general deficit in MRTF-SRF–dependent cytoskeletal gene expression, which prevents effective response to chemokine signals (Figure 7G).
Although Srf-dependent gene expression is strongly associated with cytoskeletal gene categories in both HSC/P cells and MEFs, barely 10% of the hundreds of Srf-dependent genes are shared between the 2 cell types. A similar result was obtained in macrophages.24 It is therefore probable that the impaired cytoskeletal dynamics associated with Srf inactivation in diverse biological contexts reflects generally defective cytoskeletal gene expression, as opposed to effects on ubiquitously expressed cytoskeletal genes.
Why is MRTF-SRF signaling specifically required for colonization of bone, but not other hematopoietic sites, by HSC/Ps? Previous studies have shown that inactivation of integrin β1, the RacGEFs Tiam1 and Vav, and the small GTPases Rac1 and Rac2 also result in defective adhesive and migratory responses to SDF-1, but in contrast to the MRTFs and SRF, these proteins are required for colonization of not only bone but also other hematopoietic tissues such as fetal liver and spleen.36-41 A trivial explanation might be that MRTF-SRF signaling controls genes required specifically for colonization of bone by HSC/Ps, and that the multiple deficits in adhesion and motility seen in these studies may simply not be relevant to colonization of other hematopoietic sites (Figure 7G, model 1). A more parsimonious, quantitative view is that only in the context of bone colonization does cytoskeletal gene expression become limiting in the absence of MRTF-SRF signaling (Figure 7G, model 2). Given the ubiquitous involvement of MRTF-SRF signaling in regulation of contractile and ECM genes,15,17 an attractive possibility is that HSC/Ps require greater contractile or degradative activity to reach and be retained at their niche in the more rigid environment of developing bone than in softer tissues such as liver. We are currently testing this idea.
Although fetal liver HSC/Ps lacking Srf reconstitute hematopoiesis in engraftment experiments, these require high irradiation doses, which both increase vascular permeability and promote chemokine release,3 and ultimately fails in serial transplantation experiments. SrfKOH cells are ineffective in competitive engraftment experiments, suggesting that they cannot compete with radio-resistant wild-type cells for retention in the stem-cell niche, another function that is SDF-1–dependent.10 Similar engraftment defects occur in adult bone marrow HSC/Ps upon acute inactivation of Srf.23 These results suggest that recently developed inhibitors of actin signaling to SRF such as CCG-142342,43 might be used therapeutically for mobilization of HSC/Ps. Thymic reconstitution by SrfKOH fetal liver cells was very inefficient, presumably reflecting defective bone marrow engraftment; the reconstituted thymuses contained reduced numbers of early thymic progenitors (ETPs), as did the SrfKOH embryonic thymus. This may reflect a defect in homing and retention of ETPs, which is also dependent on chemokine signaling through the CCR7, CCR9, and CXCR4/SDF-1 receptors.31,32,44 Moreover, although Srf is essential for thymocyte positive selection,19,20 our results show that it is not required before the DP stage of thymocyte development.
MRTF-SRF signaling is required for effective platelet formation.21 Although our work did not address this directly, we note that SrfKOH animals die perinatally with extensive hemorrhage, a phenotype seen in Nfe2−/− mice, which completely lack platelets.45 Perinatal hemorrhage may reflect a more complete block to platelet formation in SrfKOH animals, but could also arise from Srf inactivation in other hematopoietic cell types. While this manuscript was in preparation, others reported preliminary findings that hematopoietic Srf inactivation induces profound anemia22 : we suspect this may reflect the bone marrow colonization phenotype that we see, but we have been unable to obtain sufficient SrfKOH newborns to confirm this directly.
SRF cooperates with 2 families of regulatory protein partners, the rho-actin–controlled MRTFs and the MAP kinase–controlled TCFs.16 Our previous studies with animals lacking all 3 TCFs showed that these cells efficiently reconstitute hematopoiesis in transplant models, suggesting that TCF-SRF signaling is not required for bone marrow colonization.18 Instead, the bone marrow colonization and SDF-1 migratory phenotypes characteristic of SrfKOH animals are also seen in animals deleted for both Mrtfa and Mrtfb in hematopoietic cells, and thus reflect defective actin–based signaling. Inactivation of both Mrtfa and Mrtfb was required to block bone marrow colonization, although Mrtfa null animals appeared partially defective. We found that a single copy of either Mrtfa or Mrtfb in the hematopoietic system suffices to rescue viability. Thus, as was previously observed in megakaryocyte differentiation and other developmental systems,21,27 in HSC/Ps the 2 MRTFs appear to exhibit effective functional redundancy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the following for mouse strains: Srff/f, Dominique Daegelen and David Tuil (Institut Cochin, Paris), Mrtfa−/−, Mrtfbf/+, Eric Olson (University of Texas Southwestern Medical Center, Dallas), vav-iCre, Dimitris Kioussis (National Institute for Medical Research, London); Dov Zipori (Weizmann Institute, Rehovot) for MBA-2.1 endothelial cells; Derek Davies and the London Research Institute (LRI) FACS facility for flow cytometry; Aengus Stewart and Stuart Horswell (LRI Bioinformatics and Biostatistics facility) for RNA-seq data processing; Nik Matthews and the LRI Advanced Sequencing Laboratory for sequencing; Dominic Alibhai for image processing; and members of the laboratory, Dominique Bonnet and Erik Sahai for helpful comments on the manuscript.
This study was funded by Cancer Research UK core funding to the LRI and an Advanced Grant from the European Research Council (268690) (R.T.).
Authorship
Contribution: P.C. conceived the project; P.C., C.E., M.S., F.A.-A., and D.M. designed, performed, and interpreted experiments; C.E. conducted the RNA-seq analysis; M.S. constructed mouse strains and prepared cells for analysis; K.F. performed calvaria imaging experiments; R.T. designed and interpreted experiments; and P.C. and R.T. drafted the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Richard Treisman, CRUK London Research Institute, 44 Lincoln’s Inn Fields, London, WC2A 3LY, United Kingdom; e-mail: richard.triesman@cancer.org.uk.


![Figure 5. SrfKOH HSC/P cells exhibit defective adhesion, polarization, and motile responses to SDF-1. (A) Adhesion analysis. Srf+ and SrfKOH fetal liver LSK cells were mixed 1:1 and seeded on fibronectin-coated substrate (i) or on monolayers of MBA-2.1 endothelial cells (ii), in the presence or absence of 100 ng/mL SDF-1. Solid symbols, Srf+; open symbols, SrfKOH. See supplemental Figure 6D. (B) Morphology analysis. Cells were plated as in (A) on fibronectin-coated or MBA-2.1 endothelial cell monolayers, allowed to settle, and then stimulated for 45 minutes with SDF-1 before fixing and visualization with either Texas Red phalloidin (FN) or by prestaining with CFSE (MBA-2.1). Cell shape, defined as circularity = 4π (area/perimeter2) was measured. A perfect circle has circularity 1.0, which decrease with increasing elongation. Solid symbols, Srf+; open symbols, SrfKOH. See supplemental Figure 7A. (C) Transendothelial migration. CFSE-stained LSK cells were plated on SNARF-stained MBA-2.1 monolayers and treated as in (B). Panel Ci, cell locations were displayed by cumulative CFSE pixel intensity (LSKs, black lines) and [1-cumulative pixel intensity] (MBA-2.1, red line) relative to the membrane surface. Panel Cii, representative images: LSK cells in green and MBA-2.1 cells in semitransparent red. (D) Cell migration. LSK cells were plated on MBA2.1 cells and allowed to adhere for 20 minutes, and SDF-1 was added, followed by tracking for 20 minutes. The rose plots map the movement of each cell during the course of the experiment relative to its position at time zero. Data from 243 Srf+ and 228 SrfKOH LSK cells tracked in 3 independent experiments are summarized.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/8/10.1182_blood-2014-08-595603/5/m_1244f5.jpeg?Expires=1770074160&Signature=XIQyMt0RyL8k1zpseJICrXkdNjkgWuwhoVfTHTt0nDfocvMQAlGoc3JOxyMAyqleY9b7qt5uqswgKFjAVCkChVg14BEKytQJSfEk-XnLpG500bFTVgBt7Rigql8re3-9A4UkQBsPnLBk504tzzWRe05CCrlBdjc2wxLr8I-EKVB9f59SJgTqxUg6EAbf692jNa9j~NvnmsfBbBjFt8MGLG-YV1dr-gqHZoteY4gzyu1kOuMtDll~HJjuZW-4Aeu-puxhkA3TLshIjursMGdypSuPFDn9xLkdx25CL~MVHqTJnEpY8Ti5BYslwMnJcyn7tSED1qE83vkvyDMEumUgiQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 5. SrfKOH HSC/P cells exhibit defective adhesion, polarization, and motile responses to SDF-1. (A) Adhesion analysis. Srf+ and SrfKOH fetal liver LSK cells were mixed 1:1 and seeded on fibronectin-coated substrate (i) or on monolayers of MBA-2.1 endothelial cells (ii), in the presence or absence of 100 ng/mL SDF-1. Solid symbols, Srf+; open symbols, SrfKOH. See supplemental Figure 6D. (B) Morphology analysis. Cells were plated as in (A) on fibronectin-coated or MBA-2.1 endothelial cell monolayers, allowed to settle, and then stimulated for 45 minutes with SDF-1 before fixing and visualization with either Texas Red phalloidin (FN) or by prestaining with CFSE (MBA-2.1). Cell shape, defined as circularity = 4π (area/perimeter2) was measured. A perfect circle has circularity 1.0, which decrease with increasing elongation. Solid symbols, Srf+; open symbols, SrfKOH. See supplemental Figure 7A. (C) Transendothelial migration. CFSE-stained LSK cells were plated on SNARF-stained MBA-2.1 monolayers and treated as in (B). Panel Ci, cell locations were displayed by cumulative CFSE pixel intensity (LSKs, black lines) and [1-cumulative pixel intensity] (MBA-2.1, red line) relative to the membrane surface. Panel Cii, representative images: LSK cells in green and MBA-2.1 cells in semitransparent red. (D) Cell migration. LSK cells were plated on MBA2.1 cells and allowed to adhere for 20 minutes, and SDF-1 was added, followed by tracking for 20 minutes. The rose plots map the movement of each cell during the course of the experiment relative to its position at time zero. Data from 243 Srf+ and 228 SrfKOH LSK cells tracked in 3 independent experiments are summarized.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/8/10.1182_blood-2014-08-595603/5/m_1244f5.jpeg?Expires=1770074161&Signature=RHeVlxHwkGap9Fkq5An1q8cOtB4dOBO8-9~kFux5~RkBP-26zH3yts71cMvlHoTG95cBEmSSso4lCszJ~Uzx6WresakYNT2cdcigfZo1ZcJEkucpUxz-cLaIL-PYxlwJbR7W5Po5yMNzNhRs-XDFaEkQC-nI57HIz-f5YorQxahR~QcOaHaJUOLSgASGdQNXmlCtPY99jKoSK8Gdu2M6yZnY2VyUbREAHMoTh33eEqhUCnKdysHtlAm7~Sidpb9vK-sqgaPRNS~WjLK4cIizdnqtIb~1VaX1cX6TJATFSjyldHrgVVZpQ0zcQqjC02PTvX57vIB-1uUugXWUFhIsFw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)