Key Points
Topical resiquimod is a safe, effective therapy for early-stage CTCL that can clear both treated and untreated skin lesions.
Responding patients had T-cell recruitment into skin, enhanced T-cell effector functions, and eradication of the malignant T-cell clones.
Abstract
Early-stage cutaneous T-cell lymphoma (CTCL) is a skin-limited lymphoma with no cure aside from stem cell transplantation. Twelve patients with stage IA-IIA CTCL were treated in a phase 1 trial of 0.03% and 0.06% topical resiquimod gel, a Toll-like receptor 7/8 agonist. Treated lesions significantly improved in 75% of patients and 30% had clearing of all treated lesions. Resiquimod also induced regression of untreated lesions. Ninety-two percent of patients had more than a 50% improvement in body surface area involvement by the modified Severity-Weighted Assessment Tool analysis and 2 patients experienced complete clearing of disease. Four of 5 patients with folliculotropic disease also improved significantly. Adverse effects were minor and largely skin limited. T-cell receptor sequencing and flow cytometry studies of T cells from treated lesions demonstrated decreased clonal malignant T cells in 90% of patients and complete eradication of malignant T cells in 30%. High responses were associated with recruitment and expansion of benign T-cell clones in treated skin, increased skin T-cell effector functions, and a trend toward increased natural killer cell functions. In patients with complete or near eradication of malignant T cells, residual clinical inflammation was associated with cytokine production by benign T cells. Fifty percent of patients had increased activation of circulating dendritic cells, consistent with a systemic response to therapy. In summary, topical resiquimod is safe and effective in early-stage CTCL and the first topical therapy to our knowledge that can induce clearance of untreated lesions and complete remissions in some patients. This trial was registered at www.clinicaltrials.gov as #NCT813320.
Introduction
Cutaneous T-cell lymphomas (CTCL) are a heterogeneous collection of non-Hodgkin lymphomas derived from T cells that traffic to the skin.1,2 Distinct clinical subtypes of CTCL include mycosis fungoides (MF), in which patients present with skin-limited disease consisting of inflammatory patches and plaques, and leukemic CTCL, in which malignant T cells accumulate in the skin, blood, and lymph nodes.3,4 Although approximately 80% of early-stage CTCL (MF) patients have a normal life expectancy, approximately 20% of patients progress to more aggressive disease, which can include development of skin tumors and systemic metastases.5 The only potentially curative therapy for both advanced MF and leukemic CTCL is stem cell transplantation.6 MF is a lifelong disease, even in patients who do not develop progressive disease. Topical steroids, light therapy, and other skin-directed therapies suppress the disease but skin lesions commonly recur following discontinuation of therapy. A curative therapy is needed, both to eradicate disease when it is still manageable in patients who will progress and to spare patients with stable disease from lifelong skin-directed therapies that can weaken the immune system and put patients at increased risk for skin cancer.
Substantial emerging data indicate that host antitumor immunity plays a critical role in controlling CTCL disease progression. For example, the beneficial effects of recombinant interleukin-12 (IL-12) in CTCL are likely mediated through the induction of cellular immunity and cytotoxic T-cell responses.7,8 The imidazoquinolines are a class of small organic molecules with potent antiviral and anticancer activities. Imiquimod, a Toll-like receptor 7 (TLR7) agonist, is Food and Drug Administration–approved for the topical treatment of genital warts, basal cell carcinomas, and low-risk squamous cell carcinomas of the skin; there have also been reports of efficacy in cutaneous metastases of malignant melanoma, invasive squamous cell carcinomas, and MF.9-11 Imiquimod induces production of multiple inflammatory cytokines, including interferon-α (IFN-α), tumor necrosis factor-α (TNF-α), IL-1α, IL-6, and IL-8, from human plasmacytoid dendritic cells (PDCs), the only human dendritic cell (DC) population that expresses TLR7.12,13 PDCs are frequent in inflamed skin and skin cancers but are rare in healthy skin.9,14 In human basal cell carcinoma, the lack of PDCs in tumors was associated with imiquimod treatment failure.14 Resiquimod is an imidazoquinoline with potent TLR7 and TLR8 stimulating activity.13 In humans, TLR8 is expressed by myeloid-derived DCs, the dominant population of DCs in healthy and inflamed human skin; resiquimod but not imiquimod potently activates these cells.13,15 Given resiquimod’s ability to stimulate DC in both healthy and inflamed skin, we chose this medication to test in the treatment of CTCL.
We describe here a phase 1 trial of 0.06% and 0.03% topical resiquimod gel applied to a limited number of skin lesions in patients with stage IA-IIA CTCL. Resiquimod had high clinical response rates, even in refractory early-stage patients, and some patients also had regression of untreated lesions. Translational studies demonstrated reduction in the malignant T-cell clones in 90% of patients and complete eradication of malignant T cells from the studied lesions in 30% of patients. High-responding patients had recruitment and expansion of benign T-cell clones in treated lesions and activation of T cells and natural killer (NK) cells in the skin.
Methods
Human subjects
All studies were conducted in accordance with the Declaration of Helsinki and approved by the University of Pennsylvania’s Institutional Review Board and the Institutional Review Board of the Partners Human Research Committee (Partners Research Management). Written consent was obtained from all patients before study entry and sample collection. Patients who entered into the study met the World Health Organization-European Organization of Research and Treatment of Cancer (EORTC) definition of MF, and to be eligible to participate were required to have early-stage CTCL (IA-IIA) in accordance with the Tumor-Node-Metastasis-Blood 2007 and EORTC revised classification system. Each was assigned a unique study number permitting the study principal investigator and study coordinator to have access to patient historical information. J.G. and A.T. analyzed the data and all authors had access to primary clinical trial data.
Study procedures and treatment
This was an open-label phase 1 trial with the objective of exploring the safety and efficacy of 2 concentrations of resiquimod gel (0.06% and 0.03%) applied to lesions of early-stage (IA, IB, IIA) CTCL. Subjects applied up to 500 mg of study drug per day to up to 4 target lesions in the 0.06% group or to 5 target lesions in the 0.03% group covering a surface area of up to 100 cm2. No other lesions were treated. Dosing was started at 3 times per week, and patients were evaluated every 2 weeks. Dosing frequency (1, 2, 3, 5, or 7 times per week) was adjusted in a stepwise manner every 2 weeks based on physician assessment of tolerability (maintained, increased, decreased with or without a dosing interruption). Resiquimod was applied for 8 weeks followed by a 4-week no-treatment period to allow resiquimod-induced inflammation to resolve so that accurate skin scores could be obtained. Subjects were permitted to undertake a second 8-week course of treatment followed by a 4-week no-treatment period and a final evaluation at week 24. Before study entry, subjects underwent a 4-week washout period during which current CTCL therapies were discontinued. During the trial, other than the study drug, the only topical therapies permitted were nonmedicated moisturizing creams. Oral antihistamines were permitted for itching. Corticosteroids of all types were forbidden, as was exposure to sunshine or ultraviolet (UV) light. Additional information regarding the conduct of the study is included in the supplemental Data, available on the Blood Web site.
Patient safety and efficacy assessments
Physical examinations were performed on every 2 weeks until study completion. Blood testing included complete blood counts and comprehensive chemistry analysis every 4 weeks throughout the study. Evaluation of efficacy was based upon improvement from baseline of the Composite Assessment of Index Lesions Disease Severity score (CAILS), which evaluates treated target lesions for size, erythema, scaling and lesion elevation, and the Severity-Weighted Assessment Tool score ( SWAT), which evaluates the entire skin surface for percent involvement with a weighted score based upon whether a lesion is a patch, plaque, or tumor. Skin biopsies were obtained at baseline and at 8 weeks from a single treated target lesion. Blood was obtained at baseline and every 2 weeks.
DNA isolation from skin
DNA was isolated from frozen, OCT-embedded or formalin fixed, paraffin-embedded (FFPE) skin samples using the QIAamp DNA Mini Kit (Qiagen) per the manufacturer's instructions with overnight tissue digestion. For FFPE samples, paraffin was first removed by 2 rounds of xylene extraction followed by 2 ethanol washes before overnight tissue digestion. Extra proteinase K was added after overnight digestion if visible tissue still remained.
High-throughput sequencing analyses
For each DNA sample, T-cell receptor-β (TCR-β) third complementarity-determining regions (CDR3) regions were amplified and sequenced using ImmunoSEQ (Adaptive Biotechnologies, Seattle, WA) from 100 to 400 ng of DNA template.16-18 Frozen and FFPE tissues gave comparable results (supplemental Figure 2).
DC and T-cell flow cytometry analyses
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll centrifugation. T cells were isolated from skin by short-term explant culture as previously described.19,20 Cells were immunostained with directly conjugated monoclonal antibodies (BD Biosciences, eBioscience, Biolegend, Beckman Coulter, or R&D Systems). For cytokine analyses, T cells were treated for 4 hours with control medium or 50 ng/mL PMA (Sigma Aldrich) and 750 ng/mL ionomycin (Life Technologies) plus 10 μg/mL Brefeldin A (BD Biosciences). Cells were surface stained, fixed, permeabilized, stained with anticytokine antibodies, and then analyzed on a Becton Dickinson FACSCanto or FACSCalibur instrument. Results were analyzed using FACSDiva or FlowJo software. Resiquimod-treated DC PBMCs were cultured with 10 µg/mL resiquimod in RPMI 1640 medium with 10% fetal bovine serum, penicillin/streptomycin, and l-glutamine for 18 hours. DCs were identified by gating on CD11c+ cells.
Statistical analyses
Primary methods of data analysis included descriptive statistics (means, medians, and standard deviations). Differences between 2 sample groups were detected using the 1-tailed Wilcoxon-Mann-Whitney test, α = 0.05. For comparisons of multiple groups, a Kruskal-Wallis 1-way analysis of variance with a Bonferroni-Dunn posttest for multiple means test was used, α=0.05.
Results
Resiquimod an effective therapy for early-stage CTCL that can induce regression of both treated and untreated lesions
Fourteen patients were enrolled in the phase 1 trial, 13 commenced therapy, 1 dropped out for personal reasons, and 12 completed therapy. Ten had stage IB CTCL, one had stage IA, one had stage IIA, and 5 had folliculotropic disease. Enrolled patients had failed a mean of 6 prior therapies, with several having failed as many as 11 prior therapies (Table 1). Despite the refractory nature of their disease, 11 of 12 patients (92%) experienced significant improvement by the end of the treatment period. Resiquimod therapy was associated with migration of CD8 T cells into treated skin lesions (supplemental Figure 1). CAILS scoring was used to evaluate the responses of treated index lesions and SWAT scoring was used to evaluate overall disease burden and the response of untreated lesions.
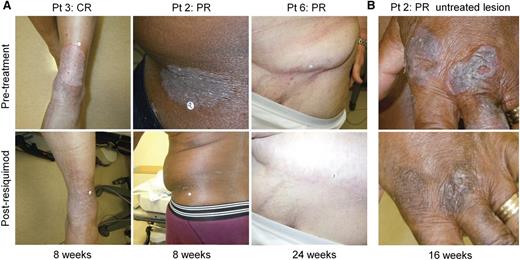
Patient 3 had stage IB CTCL with a 15-year history of inflammatory patches and plaques despite the use of 11 prior therapies. This patient experienced complete clearing of both treated and untreated skin lesions after treatment of a subset of lesions with 0.06% resiquimod (Figure 1A). Patient 2 experienced complete clearing of the treated index lesion, but only partial clearing of untreated lesions (Figure 1A-B); patient 6 had partial responses in both treated and untreated lesions (Figure 1A). When treated lesions were evaluated by CAILS scoring, 9 of 12 treated patients (75%) had significant improvement, with complete clearance in 4 of 12 treated patients and partial responses in 5 patients (Figure 2A). The remaining 3 patients also improved, but did not meet criteria for a partial response (more than 50% improvement).
Topical resiquimod therapy can induce the regression of both treated and untreated skin lesions. (A) Representative clinical images are shown of treated CTCL skin lesions before and after treatment in 3 patients. Lesions are shown before therapy (top) and at the indicated times after institution of therapy. (B) Representative clinical images of an untreated CTCL lesion before therapy (top) and at 16 weeks after beginning therapy to other sites (bottom).
Topical resiquimod therapy can induce the regression of both treated and untreated skin lesions. (A) Representative clinical images are shown of treated CTCL skin lesions before and after treatment in 3 patients. Lesions are shown before therapy (top) and at the indicated times after institution of therapy. (B) Representative clinical images of an untreated CTCL lesion before therapy (top) and at 16 weeks after beginning therapy to other sites (bottom).
Resiquimod is an effective therapy for early-stage CTCL. Changes from baseline of CAILS (A) and SWAT scores (B) are shown for the 12 patients treated. Patient numbers are shown below each bar and the dosage of topical resiquimod is indicated by bar color. Nine of 12 patients had a 50% or greater improvement from baseline of treated lesions among and 4 cleared all treated target lesions during therapy. Eleven of 12 patients experienced improvement of 50% or more in total surface area of involvement from baseline as assessed by SWAT score analysis, with 2 patients experiencing complete clearing of all evidence of disease. CR, complete remission; PR, partial remission; SD, stable disease.
Resiquimod is an effective therapy for early-stage CTCL. Changes from baseline of CAILS (A) and SWAT scores (B) are shown for the 12 patients treated. Patient numbers are shown below each bar and the dosage of topical resiquimod is indicated by bar color. Nine of 12 patients had a 50% or greater improvement from baseline of treated lesions among and 4 cleared all treated target lesions during therapy. Eleven of 12 patients experienced improvement of 50% or more in total surface area of involvement from baseline as assessed by SWAT score analysis, with 2 patients experiencing complete clearing of all evidence of disease. CR, complete remission; PR, partial remission; SD, stable disease.
Two of the 12 treated patients experienced complete clinical responses, with resolution of all evidence of disease, and 9 patients had partial responses, defined as 50% or more improvement of skin disease on the SWAT score (Figure 2B). Response rates were high for both 0.03% and 0.06% resiquimod, with complete responses occurring in the 0.06% group. Responses also appeared more rapidly among patients in the higher dose 0.06% treatment group. Four of 5 patients with folliculotropic CTCL were significantly improved at both SWAT and CAILS analyses, and 1 experienced complete clearing of all treated and untreated skin lesions.
Adverse effects of resiquimod gel were considered minor (all grade I according to the National Cancer Institute Common Toxicity Criteria) and were primarily related to local skin irritation. There were no serious adverse effects documented and none of the patients dropped out because of adverse effects of the study drug. Five of 8 patients using the 0.06% gel developed superficial skin erosions at some sites of treatment that healed completely within 1 week of temporary discontinuation of the medication. Erosions did not recur with reinitiation of topical resiquimod. Two patients using the 0.06% gel experienced 2 days of low-grade fever (below than 100.5°F) at the commencement of drug application. The remainder of the documented adverse effects (Table 1) was believed to be unrelated to the study drug.
TCR sequencing demonstrates reduced malignant clonal T cells in 90% of patients treated with topical resiquimod
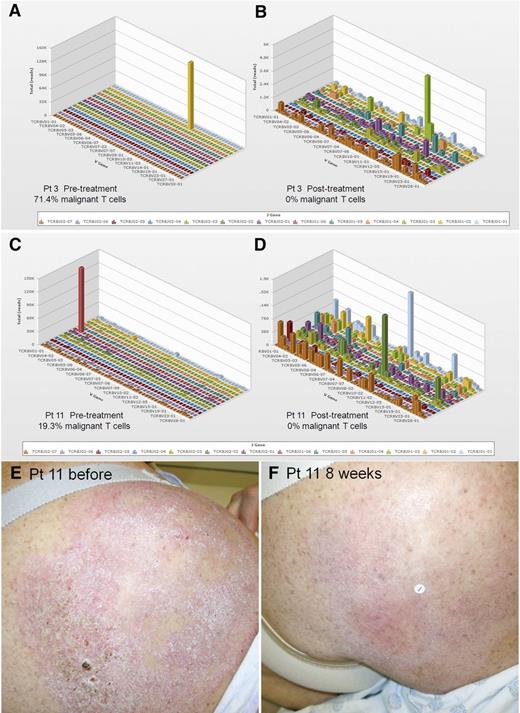
High-throughput sequencing (HTS) of the CDR3 of TCR-β genes provides comprehensive and quantitative analyses of how many distinct T-cell clones are present within a sample, the relative frequency of each clone, and the exact unique nucleotide sequences of each T-cell clone’s CDR3 region.21 DNA from biopsies of the same treated lesion, before treatment and 8 weeks after treatment, were analyzed by TCR-β HTS to identify and quantify the malignant T-cell clone (Figure 3). Of 11 patients studied, an expanded clonal T-cell population was identified in 10 (Table 2). Nine of 10 patients treated with topical resiquimod had reductions in the percentage of malignant T cells, 3 had complete eradication of the malignant clone, and a fourth had a 99.6% reduction (Figure 4A; Table 2). Improvement in skin inflammation tended to lag behind reduction or eradication of the malignant T-cell clone from treated lesions (Figure 4B).
Evaluation of benign and malignant T cells in skin lesions before and after resiquimod therapy using HTS TCR analysis. DNA was isolated from lesional skin before and after resiquimod therapy and analyzed by HTS TCR-β analysis. HTS results before (A,C) and after (B,D) resiquimod therapy for 2 patients with a complete response are shown. This technique allows identification and quantification of the malignant T-cell clone as well as measurement of the exact number, diversity, relative proportions, and sequences of all T cells in the sample. (E,F) Clinical images of a treated lesion from patient 11 are shown. V, variable.
Evaluation of benign and malignant T cells in skin lesions before and after resiquimod therapy using HTS TCR analysis. DNA was isolated from lesional skin before and after resiquimod therapy and analyzed by HTS TCR-β analysis. HTS results before (A,C) and after (B,D) resiquimod therapy for 2 patients with a complete response are shown. This technique allows identification and quantification of the malignant T-cell clone as well as measurement of the exact number, diversity, relative proportions, and sequences of all T cells in the sample. (E,F) Clinical images of a treated lesion from patient 11 are shown. V, variable.
Nine of 10 patients tested had reduction in the frequency of malignant T cells in skin following resiquimod therapy. HTS of DNA from lesional skin before therapy and at 8 weeks was used to identify and quantify clonal malignant T cells. (A) The change in frequency of the malignant T-cell clone as a percentage of the total T-cell population is shown. Patients with a clinical CR (patients 3 and 8) and PR (all others) are indicated. Patient 11, a clinical PR, had eradication of the malignant clone at 8 weeks and patient 1, a clinical PR, had a 99.6% reduction in the malignant clone. Only patient 6 showed a slight increase in the malignant T-cell frequency. (B) The correlation of clinical SWAT scores with malignant T-cell clone frequency by HTS are shown. In general, clinical improvement lagged behind clearance of the malignant T-cell clone and in a subset of patients (11, 1, 5), clinical inflammation persisted despite complete or near eradication of the malignant clone.
Nine of 10 patients tested had reduction in the frequency of malignant T cells in skin following resiquimod therapy. HTS of DNA from lesional skin before therapy and at 8 weeks was used to identify and quantify clonal malignant T cells. (A) The change in frequency of the malignant T-cell clone as a percentage of the total T-cell population is shown. Patients with a clinical CR (patients 3 and 8) and PR (all others) are indicated. Patient 11, a clinical PR, had eradication of the malignant clone at 8 weeks and patient 1, a clinical PR, had a 99.6% reduction in the malignant clone. Only patient 6 showed a slight increase in the malignant T-cell frequency. (B) The correlation of clinical SWAT scores with malignant T-cell clone frequency by HTS are shown. In general, clinical improvement lagged behind clearance of the malignant T-cell clone and in a subset of patients (11, 1, 5), clinical inflammation persisted despite complete or near eradication of the malignant clone.
Eradication of malignant T cells correlated with recruitment and expansion of new responding benign T cells but not to clonal T-cell burden or number of infiltrating T cells after therapy
We evaluated the absolute numbers of malignant T cells (Figure 5A) and benign infiltrating T cells (Figure 5B) in skin lesions before and 8 weeks after initiating therapy with topical resiquimod. The number of malignant T cells before therapy (initial malignant T-cell burden) and the total number of benign infiltrating T cells before or after therapy were not significantly different in clinical high responders (patients 3 and 8) and complete molecular responders (clearance of the malignant T cells; patients 3, 8, and 11) vs patients who responded less favorably. The numbers of expanded benign T-cell clones after therapy, defined as clones that made up more than 1% of the total T-cell population, were significantly higher in complete responders (Figure 5C). To determine if these expanded T-cell clones were resident in the skin before resiquimod therapy, we identified the top 20 benign T-cell clones after treatment and determined if these clonal T-cell sequences were also present before therapy (Figure 5D). Although differences did not reach statistical significance, improved clearance of the malignant T-cell clone was associated with recruitment of new benign T-cell clones that were not present before therapy. In resiquimod high responders, T-cell clones that were present before therapy also underwent marked expansion (Figure 5E-F; supplemental Figure 3).
Eradication of malignant T cells correlated with recruitment and expansion of new responding benign T cells, but was not related to the initial burden of malignant clonal T cells or the total number of benign T cells before or after therapy. (A) The absolute numbers of malignant T cells per 100 µg of DNA before (pre-tx) and after therapy (post-tx) are shown. There was no correlation of a complete or near-complete eradication of malignant T cells (patients 3, 8,1 1, and 1) with initial clonal T-cell burden. (B) The absolute number of benign infiltrating T cells before and after therapy are shown and did not correlate with clearance of the malignant T-cell clone. (C) Expansion of benign T-cell clones in treated lesions was associated with improved clinical responses. The number of expanded (>1% frequency) benign infiltrating T-cell clones in patients after treatment are shown and was associated with improved clinical responses and eradication of clonal malignant T cells. (D) The percentage of the top 20 benign T-cell clones that were newly recruited vs resident in the skin before treatment are shown. The 20 most frequent benign T-cell clones were identified after therapy and their presence before treatment was determined. Patients were divided into high responders (high resp; >99% malignant T-cell eradication), mid-responders (mid resp; 50% to 99% eradication), and low responders (low resp; 0% to 50% eradication). Recruitment of new responding benign T-cell clones into treated lesions was associated with better eradication of malignant T cells. (E-F) The frequency and absolute numbers of the top 10 most frequent benign T-cell clones pre-tx and post-tx are shown in examples of high-responding (HR) and low-responding (LR) patients. In general, HR patients had marked expansion of benign T-cell clones. Pt, patient.
Eradication of malignant T cells correlated with recruitment and expansion of new responding benign T cells, but was not related to the initial burden of malignant clonal T cells or the total number of benign T cells before or after therapy. (A) The absolute numbers of malignant T cells per 100 µg of DNA before (pre-tx) and after therapy (post-tx) are shown. There was no correlation of a complete or near-complete eradication of malignant T cells (patients 3, 8,1 1, and 1) with initial clonal T-cell burden. (B) The absolute number of benign infiltrating T cells before and after therapy are shown and did not correlate with clearance of the malignant T-cell clone. (C) Expansion of benign T-cell clones in treated lesions was associated with improved clinical responses. The number of expanded (>1% frequency) benign infiltrating T-cell clones in patients after treatment are shown and was associated with improved clinical responses and eradication of clonal malignant T cells. (D) The percentage of the top 20 benign T-cell clones that were newly recruited vs resident in the skin before treatment are shown. The 20 most frequent benign T-cell clones were identified after therapy and their presence before treatment was determined. Patients were divided into high responders (high resp; >99% malignant T-cell eradication), mid-responders (mid resp; 50% to 99% eradication), and low responders (low resp; 0% to 50% eradication). Recruitment of new responding benign T-cell clones into treated lesions was associated with better eradication of malignant T cells. (E-F) The frequency and absolute numbers of the top 10 most frequent benign T-cell clones pre-tx and post-tx are shown in examples of high-responding (HR) and low-responding (LR) patients. In general, HR patients had marked expansion of benign T-cell clones. Pt, patient.
Malignant T-cell eradication is associated with increased T-cell effector functions in treated skin
We extracted NK and T cells from skin lesions before and 8 weeks after resiquimod therapy was initiated and analyzed them by flow cytometry for surface phenotype and effector functions. Six patients were studied, 3 high responders (patients who cleared >99% of the malignant clone; patients 1, 3, and 8) and 3 lower responders (patients 4-6). Production of IFN-γ and TNF-α by CD4+ T cells was significantly greater in high vs low responders (Figure 6A-D), whereas IFN-γ and TNF-α production by CD8+ T cells was high in both groups. Production of granzyme B by CD8+ T cells was also significantly higher in high- vs low-responding patients and perforin was increased but was not statistically significant (Figure 6D). Other parameters, including % CD8 T cells, % FOXP3+ regulatory T cells, CD107 expression, and production of IL-17 and IL-22, were not significantly different between high- and low-responding patients (data not shown). High-responding patients tended to have higher production of IFN-γ, perforin, and granzyme by CD56+ NK cells, although low sample numbers prevented these differences from being statistically significant.
Malignant T-cell eradication is associated with increased T-cell and NK-effector functions in treated skin. NK and T cells were extracted from lesional skin before and at 8 weeks after resiquimod therapy in 3 HR (1, 3, and 8) and 3 LR (4-6) patients and production of cytokines and effector molecules was evaluated by intracellular cytokine staining and flow cytometry analysis after PMA and ionomycin stimulation. (A-C) Malignant clonal T-cell eradication was associated with high levels of IFN-γ production by both CD4+ and CD8+ T cells. (A) IFN-γ and IL-17 production by CD4+ T cells from patient 3, who had eradication of malignant T cells and a complete clinical response, is shown. Individual patients, before and after resiquimod therapy (B) and aggregate measurements after resiquimod therapy (C) of T-cell IFN-γ production are shown. CD4+ T-cell production of IFN-γ was significantly higher in HR patients. (D) Production of TNF-α, perforin, and granzyme by CD4+ and CD8+ T cells after therapy is shown. Production of TNF-α by CD4+ T cells and granzyme by CD8+ T cells was significantly higher in HR patients. (E) Increased NK effector functions were associated with clearance of the malignant clone. Production of IFN-γ, perforin, and granzyme by CD56+ NK cells is shown. There was a trend for increased NK effector functions in HR patients but given the low number of samples, these differences were not significant. *P < .05, **P < .01.
Malignant T-cell eradication is associated with increased T-cell and NK-effector functions in treated skin. NK and T cells were extracted from lesional skin before and at 8 weeks after resiquimod therapy in 3 HR (1, 3, and 8) and 3 LR (4-6) patients and production of cytokines and effector molecules was evaluated by intracellular cytokine staining and flow cytometry analysis after PMA and ionomycin stimulation. (A-C) Malignant clonal T-cell eradication was associated with high levels of IFN-γ production by both CD4+ and CD8+ T cells. (A) IFN-γ and IL-17 production by CD4+ T cells from patient 3, who had eradication of malignant T cells and a complete clinical response, is shown. Individual patients, before and after resiquimod therapy (B) and aggregate measurements after resiquimod therapy (C) of T-cell IFN-γ production are shown. CD4+ T-cell production of IFN-γ was significantly higher in HR patients. (D) Production of TNF-α, perforin, and granzyme by CD4+ and CD8+ T cells after therapy is shown. Production of TNF-α by CD4+ T cells and granzyme by CD8+ T cells was significantly higher in HR patients. (E) Increased NK effector functions were associated with clearance of the malignant clone. Production of IFN-γ, perforin, and granzyme by CD56+ NK cells is shown. There was a trend for increased NK effector functions in HR patients but given the low number of samples, these differences were not significant. *P < .05, **P < .01.
TCR sequencing can be more specific than clinical score evaluation in assessing malignant T-cell clone clearance
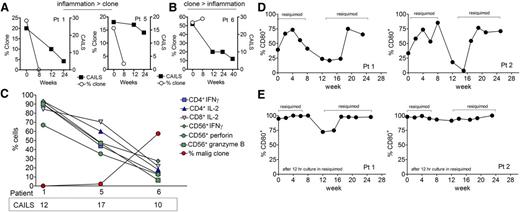
Although in many patients, clinical scores improved following reduction of the malignant T-cell clone, at least 3 patients (1, 5, and 11) still had clinically evident disease despite near-complete eradication of malignant T cells (Figure 7A). In addition, the 1 patient who had an increase in the frequency of the malignant T-cell clone after therapy appeared clinically to have improved (Figure 7B). We correlated clinical inflammation of the treated lesion, as assessed by CAILS scores, with the malignant T-cell burden and benign T- and NK-cell production of inflammatory cytokines and effector molecules. We observed that residual inflammation on clinical examination was more reflective of benign T-cell activation than the presence of malignant T cells (Figure 7C). However, only 1 skin lesion was studied by HTS for each patient and results in this lesion do not necessarily reflect the numbers of malignant T cells in other lesions.
TCR sequencing is superior to clinical score evaluation in assessing clearance of the malignant T-cell clones and resiquimod is associated with maturation of circulating DCs. (A) Although in many patients, clinical scores improved following reduction of the malignant T cell clone, at least 3 patients (1, 5, and 11) still had clinically evident disease despite near-complete eradication of malignant T cells. The percentage malignant clone and clinical CAILS scores for patients 1 and 5 are shown. (B) Likewise, improvement in clinical score did not necessarily reflect depletion of malignant T cells. Results for patients 6 are shown, in whom the percentage and absolute number of malignant T cells increased despite a clinically improving examination. (C) Residual inflammation on clinical examination is more reflective of benign T-cell activation than the presence of malignant T cells. The CAIL clinical scores at 12 weeks, the percentage of benign T and NK cells making the indicated cytokines, and the percentage of malignant clonal T cells are shown together for patients 1, 5, and 6. In patients 1 and 5, activation of benign T cells was evident despite near-complete eradication of the malignant T-cell clone, suggesting benign T cells were producing the inflammation measured by clinical CAILS scores. In patient 6, low cytokine production by benign T cells was associated with an improving clinical score but an increasing burden of malignant T cells. (D) CD80 expression is increased on circulating CD11c+ myeloid-derived DCs during periods of topical resiquimod use. PBMCs were isolated from peripheral blood at the indicated time points and expression of CD80 on CD11c+ DC was assessed by flow cytometry. Periods when patients were applying resiquimod gel are indicated. Representative results from 2 patients are shown; a similar pattern of DC maturation was observed in 4/8 patients tested. (E) Resiquimod further enhanced DC maturation in vitro. PBMCs isolated from blood at the indicated time points were incubated overnight in 10 µg/mL resiquimod and surface expression of CD80 on CD11c+ DCs was assessed by flow cytometry.
TCR sequencing is superior to clinical score evaluation in assessing clearance of the malignant T-cell clones and resiquimod is associated with maturation of circulating DCs. (A) Although in many patients, clinical scores improved following reduction of the malignant T cell clone, at least 3 patients (1, 5, and 11) still had clinically evident disease despite near-complete eradication of malignant T cells. The percentage malignant clone and clinical CAILS scores for patients 1 and 5 are shown. (B) Likewise, improvement in clinical score did not necessarily reflect depletion of malignant T cells. Results for patients 6 are shown, in whom the percentage and absolute number of malignant T cells increased despite a clinically improving examination. (C) Residual inflammation on clinical examination is more reflective of benign T-cell activation than the presence of malignant T cells. The CAIL clinical scores at 12 weeks, the percentage of benign T and NK cells making the indicated cytokines, and the percentage of malignant clonal T cells are shown together for patients 1, 5, and 6. In patients 1 and 5, activation of benign T cells was evident despite near-complete eradication of the malignant T-cell clone, suggesting benign T cells were producing the inflammation measured by clinical CAILS scores. In patient 6, low cytokine production by benign T cells was associated with an improving clinical score but an increasing burden of malignant T cells. (D) CD80 expression is increased on circulating CD11c+ myeloid-derived DCs during periods of topical resiquimod use. PBMCs were isolated from peripheral blood at the indicated time points and expression of CD80 on CD11c+ DC was assessed by flow cytometry. Periods when patients were applying resiquimod gel are indicated. Representative results from 2 patients are shown; a similar pattern of DC maturation was observed in 4/8 patients tested. (E) Resiquimod further enhanced DC maturation in vitro. PBMCs isolated from blood at the indicated time points were incubated overnight in 10 µg/mL resiquimod and surface expression of CD80 on CD11c+ DCs was assessed by flow cytometry.
Topical resiquimod is associated with maturation of circulating DCs
We observed regression of distant, untreated MF skin lesions in patients treated locally with topical resiquimod, arguing for induction of systemic antitumor immunity. Two patients also developed systemic symptoms of low-grade fevers following the initiation of topical resiquimod. Hypothesizing that topical application of resiquimod may lead to systemic activation of DCs, we isolated CD11c+ myeloid DCs from the peripheral blood and assayed for activation by immunostaining for CD80. We observed that DC CD80 expression rose after institution of topical resiquimod therapy, began to decline during the 4-week rest period, and then rose again with resumption of topical resiquimod therapy (Figure 7D). Similar increases in circulating myeloid DC CD80 expression were observed among 4 of 8 tested subjects. However, peripheral blood DC maturation did not correlate with increased clinical responses or with the concentration of the study drug used. Overnight culture of DCs from blood in the presence of 10 µg/mL resiquimod further enhanced CD80 expression, confirming the ability of the study drug to activate DCs (Figure 7E).
Discussion
This phase 1 trial demonstrates that topical resiquimod is safe, well-tolerated, and highly effective for the treatment of early-stage CTCL. Although the trial was small, 75% of patients had significant improvement in treated lesions by CAILS assessment, with 4 of 12 clearing all treated lesions and an additional 5 experiencing at least a 50% improvement. Remarkably, topical resiquimod also induced regression of untreated lesions, with 92% of patients having more than a 50% improvement and 2 patients clearing all evidence of disease. To our knowledge, this is the first topical therapy for CTCL that has induced regression of both treated and untreated lesions. Overall, response rates to topical resiquimod were superior to those observed with other topical therapies, including bexarotene gel, potent topical steroids, and nitrogen mustard gel.22 Results suggested the 0.06% strength was superior to the 0.03% dosage; complete responses were noted only in patients receiving the 0.06% gel, and responses in this dose group were faster, with 5 of 7 responses occurring in the high-dose group by week 12 as compared with 1 of 4 in the 0.03% group. Both doses were equally well-tolerated, with minor skin reactions representing the most common adverse effects. Given these results, further enrollment in this study was closed to facilitate the initiation of a larger phase 2 multicenter, placebo-controlled trial.
Five patients in the study had the difficult-to-control folliculotropic variant of CTCL and had progressive skin disease despite the institution of multiple prior therapies (Table 1). Remarkably, all improved; 4 of 5 had significant improvements in both CAILS and modified SWAT scores, and 1 experienced a complete remission. Two patients had intractable, generalized pruritus before therapy and both experienced complete resolution of pruritus by week 8 of treatment.
The optimal outcome of any cancer therapy is the elimination of all malignant cells. The skin lesions of early-stage CTCL contain a mixture of clonal malignant T cells and benign infiltrating T cells and there are no markers that reliably distinguish malignant from benign T cells. This has made study of early-stage CTCL and assessment of therapeutic responses difficult. We used HTS of the TCR-β gene CDR3 regions to identify the malignant T-cell clone and quantify responses to therapy. Of 11 patients sequenced, a malignant T-cell clone was identified in 10. Ninety percent of patients had reduction in the percent of the malignant T-cell clones within the biopsied lesions after therapy and 3/10 had complete eradication of the malignant T-cell clone when assessed by HTS. A fourth patient had near elimination (99.6% reduction) of malignant T cells. However, responses to therapy can differ in distant lesions and in different areas of a single treated lesion; HTS studies are representative of the response in the sampled area, but may not reflect responses in other lesions or different areas of the same treated lesion.
Treated skin lesions contain not only preexisting malignant and benign infiltrating T cells, but also T cells and other immune cells recruited to the skin by resiquimod therapy. Inflammation caused by benign infiltrating T cells and by the therapy itself can make it difficult to clinically assess the continued presence of malignant T cells. Indeed, we documented that the residual clinical inflammation observed in patients with complete or near eradication of malignant T cells was associated with the continued presence of benign T cells producing inflammatory cytokines. By virtue of its ability to directly measure the number of malignant T cells in skin, HTS was remarkably useful in assessing the responses of CTCL skin lesions to therapy.
Using a combination of HTS and flow cytometry analyses, we found that effective responses to resiquimod were associated with activation and enhanced production of IFN-γ and TNF-α by CD4+ T cells, enhanced effector functions in CD8+ T cells, and a trend toward enhanced NK function. Surprisingly, CD8+ T-cell production of IFN-γ was high in both low and high responders and did not correlate with clearance of the malignant T-cell clone. A high burden of clonal malignant T cells before therapy did not correlate with poor responses to topical resiquimod. Patient 3 had refractory CTCL for 15 years before entry into the study and HTS demonstrated that malignant T cells made up 71% of the total T-cell population in the biopsied lesion. Despite this, the patient had a complete response to topical resiquimod, with clearance of both treated and untreated lesions, and has remained clear of all disease for 1 year off all therapy. Likewise, the total number of benign T cells infiltrating CTCL skin lesions before or after therapy did not predict responsiveness to therapy. However, the presence of expanded individual benign T-cell clones in treated lesions (clones present at a frequency of 1% or more) did correlate with increased clearance of malignant T cells. Taken together, these results suggest activation of CD4+ T cells and the recruitment and expansion of responding, presumably tumor-specific T cells is critical for the eradication of the malignant T cells in CTCL skin lesions in response to resiquimod.
Regression of untreated CTCL skin lesions in multiple patients demonstrates that systemic antitumor immunity can develop after local topical resiquimod therapy. Two patients had systemic symptoms of low-grade fevers and 4 of 8 patients tested had activation of circulating CD11c+ DCs. However, DC activation was not correlated with disease clearance, suggesting that although antigen-presenting cell activation and enhanced antigen presentation may occur, a patient must still have T cells capable of recognizing and responding to the cancer to experience clearance of disease.
The current study demonstrates that topical resiquimod therapy is a remarkably effective, well-tolerated therapy for early-stage CTCL. In some patients, topical resiquimod-induced regression of both treated and untreated skin lesions, suggesting enhanced systemic antitumor immunity. These studies support further trials of this medication in early-stage, skin-limited CTCL and suggest resiquimod might also be useful as an adjuvant therapy in the treatment of more advanced CTCL. T cells from patients with advanced CTCL treated in vitro with a combination of IFN-γ and resiquimod showed enhanced T helper 1 cytokine production and effector functions, suggesting there may be utility to combining systemic IFN-γ, currently standard of care, with topical resiquimod.23 Furthermore, combination therapy using resiquimod and low-dose lesional radiation to release tumor antigens is another promising approach, as recently demonstrated by Kim and colleagues using the TLR9 agonist CpG.24 Last, our studies demonstrate that HTS is an effective and accurate method of assessing malignant T cells in skin lesions both before and after therapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients who made this work possible, the members of the Division of Orphan Products, the US Food and Drug Administration for the provision of support and guidance throughout the study, and Galderma/Spirig Pharma, Ekerkingen, Switzerland, for provision of the study drug. The authors also thank Drs Harlan Robins and Thomas S. Kupper for establishing collaboration between the Clark laboratory and Adaptive Biotechnologies and Elizabeth Lowry, Dr Christopher Elco, and Dr Jessica Teague for assistance in sample and figure preparation.
This work was supported by the National Institutes of Health (NIH)/National Cancer Institute grant R01 CA122569 (to A.H.R.), grant R21 CA178424 (to A.H.R. and M.W.), and grant FD-RO1-04092 (to A.H.R.); NIH/National Institute of Arthritis and Musculoskeletal and Skin Diseases (grants R01 AR056720 and R01 AR063962) (to R.A.C.); and NIH (R01 AI097128) (to Thomas S. Kupper and R.A.C.). This work was also supported by a CLARIONS grant from the Cutaneous Lymphoma Foundation (to R.A.C.), a Translational Research grant from the Leukemia and Lymphoma Society (to A.H.R.), and a Special Fellow grant from the Leukemia & Lymphoma Society (to R.W.).
Authorship
Contribution: A.H.R. designed, supported, and supervised the clinical trial and obtained the clinical specimens; R.A.C. designed, supported, supervised, and analyzed the experiments shown in Figures 3-7, and drafted the figures and the manuscript in collaboration with A.H.R.; J.M.G. assisted in trial design and evaluation of the clinical data; M.W. and B.B. performed and analyzed the experiments shown in Figure 7D-E; A.B.T. conducted trial design and statistical analyses and contributed to editing the manuscript; R.E. analyzed histology of skin biopsies; M.A.B. was the nurse coordinator for the clinical trial; D.S.L. performed the regulatory submissions for the clinical trial; C.S. assisted in the clinical trial dosing schedule and provided the study medications; R.W. isolated T cells and DNA from skin lesions and carried out and analyzed flow cytometry analyses; I.R.K. carried out high-throughput sequencing studies on DNA derived from patient samples; and E.J.K. assisted in patient evaluation during the clinical trial.
Conflict-of-interest disclosure: R.A.C. and I.R.K. are named as inventors on 2 pending patent applications regarding the utility of high-throughput sequencing in cutaneous lymphoma. I.R.K. holds stock in and is employed by Adaptive Biotechnologies. The remaining authors declare no competing financial interests.
Correspondence: Alain Rook, Department of Dermatology and the Center for Clinical Biostatistics and Epidemiology, Perelman School of Medicine, 3600 Spruce St, Philadelphia, PA 19104; e-mail: arook@mail.med.upenn.edu; and Rachael Clark, Department of Dermatology, Brigham and Women’s Hospital, EBRC 505A, 221 Longwood Ave, Boston, MA 02115; e-mail: rclark1@partners.org.