Key Points
NOX2-generated ROS regulate the function of surface receptors required for platelet-neutrophil interactions during vascular inflammation.
Abstract
Platelet-leukocyte interactions on activated endothelial cells play an important role during microvascular occlusion under oxidative stress conditions. However, it remains poorly understood how neutrophil-platelet interactions are regulated during vascular inflammation. By using intravital microscopy with mice lacking nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 2 (NOX2) and their bone marrow chimera, we demonstrated that NOX2 from both hematopoietic and endothelial cells is crucial for neutrophil-platelet interactions during tumor necrosis factor alpha-induced venular inflammation. Platelet NOX2-produced reactive oxygen species (ROS) regulated P-selectin exposure upon agonist stimulation and the ligand-binding function of glycoprotein Ibα. Furthermore, neutrophil NOX2-generated ROS enhanced the activation and ligand-binding activity of αMβ2 integrin following N-formyl-methionyl-leucyl phenylalanine stimulation. Studies with isolated cells and a mouse model of hepatic ischemia/reperfusion injury revealed that NOX2 from both platelets and neutrophils is required for cell-cell interactions, which contribute to the pathology of hepatic ischemia/reperfusion injury. Platelet NOX2 modulated intracellular Ca2+ release but not store-operated Ca2+ entry (SOCE), whereas neutrophil NOX2 was crucial for SOCE but not intracellular Ca2+ release. Different regulation of Ca2+ signaling by platelet and neutrophil NOX2 correlated with differences in the phosphorylation of AKT, ERK, and p38MAPK. Our results indicate that platelet and neutrophil NOX2-produced ROS are critical for the function of surface receptors essential for neutrophil-platelet interactions during vascular inflammation.
Introduction
Recent studies have provided compelling evidence that neutrophil-platelet interactions on activated endothelial cells (ECs) are the major determinant of vascular occlusion during thromboinflammatory disease in which inflammation is coupled to thrombosis.1 Once venular ECs are inflamed and activated, neutrophils roll over the endothelium through interactions between selectins and their ligands.2,3 Then, activated αLβ2 integrin mediates neutrophil adhesion to intercellular adhesion molecule-1 (ICAM-1) on activated ECs. Activated αMβ2 integrin, a dominant receptor on activated neutrophils, mainly controls neutrophil crawling on the endothelium. The I domain of the αM subunit is able to interact with numerous molecules, including ICAM-1, fibrinogen (FG), complement C3, and platelet glycoprotein Ibα (GPIbα),4-7 thereby mediating vascular disease. Because granular molecules secreted from activated neutrophils enhance prothrombotic responses,4 activated neutrophils adhered to inflamed ECs could provide an adhesive surface that promotes platelet adhesion and accumulation.1,8
Heterotypic neutrophil-platelet interactions are mainly mediated by binding of platelet P-selectin and GPIbα to neutrophil P-selectin glycoprotein ligand-1 (PSGL-1) and αMβ2 integrin, respectively.2 In particular, the interaction between GPIbα and αMβ2 integrin is required for stable and firm attachment of platelets to neutrophils.7 Although the major receptors and counter-receptors are well identified, it remains unclear how heterotypic cell-cell interactions are modulated during vascular inflammation. Recently, we demonstrated that neutrophil AKT2 plays a critical role during membrane translocation and activation of αMβ2 integrin, thereby mediating neutrophil-platelet interactions under thromboinflammatory conditions.1 It was reported that neutrophil AKT2, but not AKT1, translocates to the leading edge of the plasma membrane after agonist stimulation and stimulates nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 2 (NOX2) activity.9 Although reactive oxygen species (ROS) are important regulators during vascular disease,10,11 little is known about whether and how ROS influence neutrophil-platelet interactions during vascular inflammation.
NOX1, NOX2, and NOX4 are expressed in intravascular cells in both humans and mice.12 However, NOX5 is expressed only in the vasculature of humans. Compared with platelets that produce low amounts of ROS through NOX1 and NOX2,13,14 neutrophils rapidly generate much larger amounts of ROS via NOX2 after cell activation.15 The NOX2 enzyme consists of membrane subunits (p22phox and gp91phox) and cytosolic components (p47phox, p67phox, p40phox, and small GTPase Rac1/2), and generates O2·- by transferring 1 electron from NADPH to molecular oxygen.12 Upon agonist stimulation, some cytosolic components are phosphorylated and translocated to the plasma membrane, where the NOX2 complex is assembled. NOX2-generated O2·- is rapidly converted into longer-lasting and membrane diffusible H2O2, which is the major ROS contributing to pathological signaling through oxidative modification of lipids and proteins.11
Previous studies showed that glycoprotein VI (GPVI)-mediated platelet aggregation and ROS generation are significantly impaired by pretreatment with nonselective NOX inhibitors and ROS scavengers.13,16 Platelets from patients deficient in gp91phox (X-linked chronic granulomatous disease [X-CGD]) showed defects in CD40 ligand expression induced by various agonists and production of ROS.17 Furthermore, clinical studies with X-CGD patients demonstrated that NOX2 deficiency increases serum levels of nitrite and nitrate as markers of nitric oxide generation, thereby enhancing arterial dilation.18 Neutrophil NOX2-generated ROS are crucial for killing microbial pathogens19 and function as signaling molecules that regulate the activity of kinases and phosphatases.20 Thus, NOX2 is a main source for extracellular ROS generation and regulates platelet and neutrophil functions.
In this study, we investigated the role of NOX2 in regulating neutrophil-platelet interactions during vascular inflammation. We found that platelet and neutrophil NOX2-derived ROS regulate the function of surface receptors, thereby inducing the cell-cell interactions during vascular inflammation. Our results provide mechanistic insight into how neutrophil-platelet interactions are regulated under oxidative stress.
Materials and methods
Mice
Wild-type (WT) and homozygous NOX2 knockout (KO) mice with a C57BL/6 background (6 to 10 weeks old) were obtained from the Jackson Laboratory (Bar Harbor, ME). Except for intravital microscopy, both sexes were used in all studies. The University of Illinois Institutional Animal Care and Use Committee approved all animal care and experimental procedures.
Isolation of platelets and neutrophils
Mouse and human platelets and neutrophils were prepared as described previously.1,21,22 Washed platelets were suspended in N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid-Tyrode buffer (20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid [pH 7.3], 136 mM NaCl, 2.7 mM KCl, 12 mM NaHCO3, 1 mM MgCl2, and 5.5 mM glucose) at a concentration of 3 × 108 cells per milliliter. The concentrations of neutrophils were adjusted to 1 × 107 cells per milliliter. Human and mouse neutrophils were stimulated with 0.5 and 10 µM N-formyl-methionyl-leucyl phenylalanine (fMLF), respectively, for 10 minutes at 37°C, unless otherwise stated.
Soluble αMβ2 binding to platelets
Platelets (3 × 106) were pretreated with or without catalase (1000 U/mL) for 10 minutes or an anti-human GPIbα antibody (VM16d, 1 μg/mL) for 30 minutes. Cells were then treated with recombinant human αMβ2 (10 µg/mL) in the presence or absence of 0.5 mM MnCl2 for 1 hour at 37°C. After washing out unbound αMβ2, platelets were incubated with 5 µg/mL fluorescein isothiocyanate-conjugated control immunoglobulin G or goat anti-human β2 antibodies, followed by flow cytometric analysis.
Intravital microscopy
Intravital microscopy was performed in a mouse model of tumor necrosis factor alpha (TNF-α) -induced cremaster venular inflammation as described in supplemental Data available on the Blood Web site.1 The number of rolling and adherent neutrophils was normalized to both neutrophil count and vessel length. Adhesion and accumulation of platelets on adherent neutrophils were determined in the integrated median fluorescence intensities of the anti-CD42c (GPIbβ) antibody, which was normalized to the number of adherent neutrophils and plotted as a function of time.
Statistics
Data were analyzed by using GraphPad Prism software. Statistical significance was assessed by analysis of variance (ANOVA) and Dunnett’s test, Student t test, or Mann-Whitney test. A P value less than .05 was considered significant.
Additional methods
Detailed methods regarding bone marrow transplantation, flow cytometry, von Willebrand factor (vWF) or FG binding assay, platelet agglutination assay, ROS generation, in vitro neutrophil-platelet aggregation, Ca2+ mobilization, immunoprecipitation, and hepatic ischemia/reperfusion injury are presented in supplemental Data.
Results
Platelet and neutrophil NOX2 play critical role in regulating neutrophil-platelet association during TNF-α-induced venular inflammation
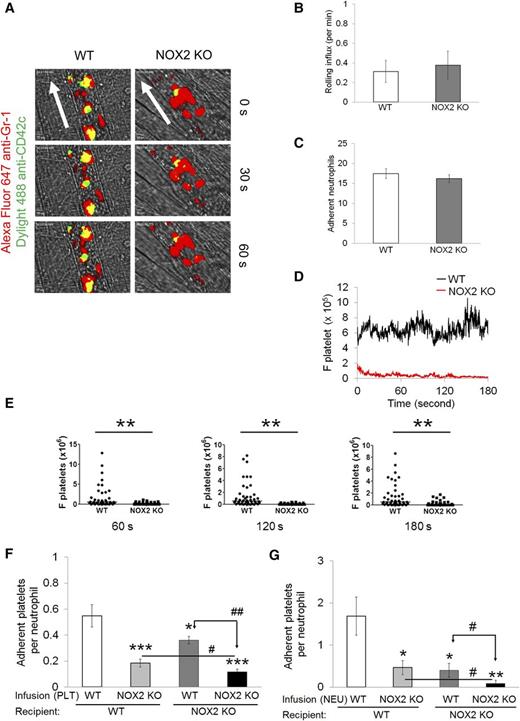
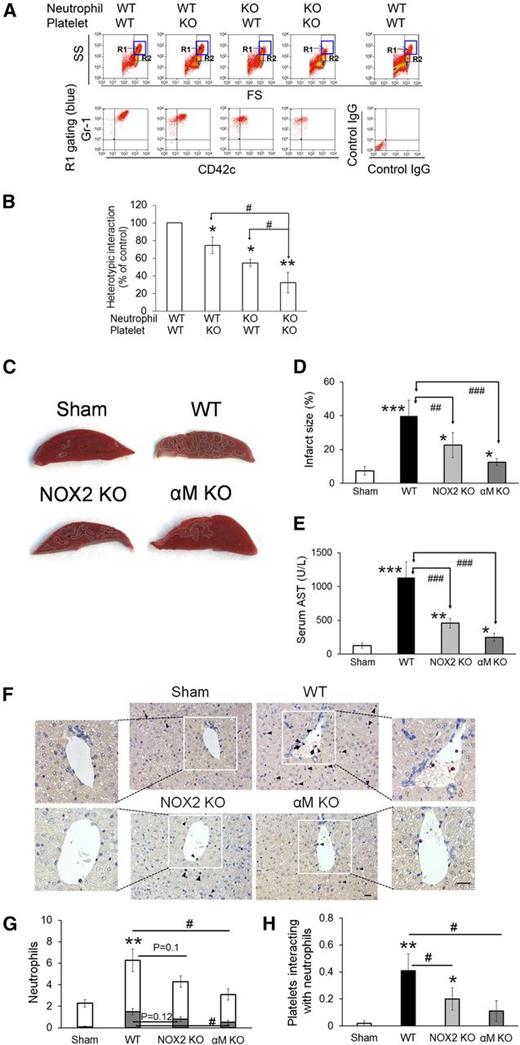
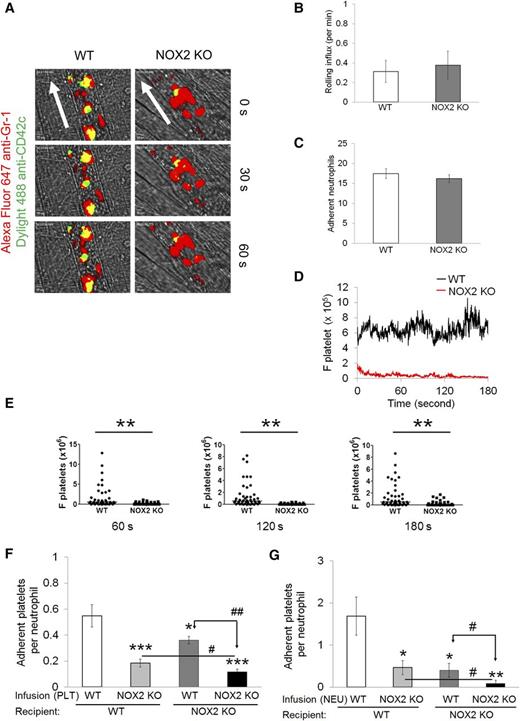
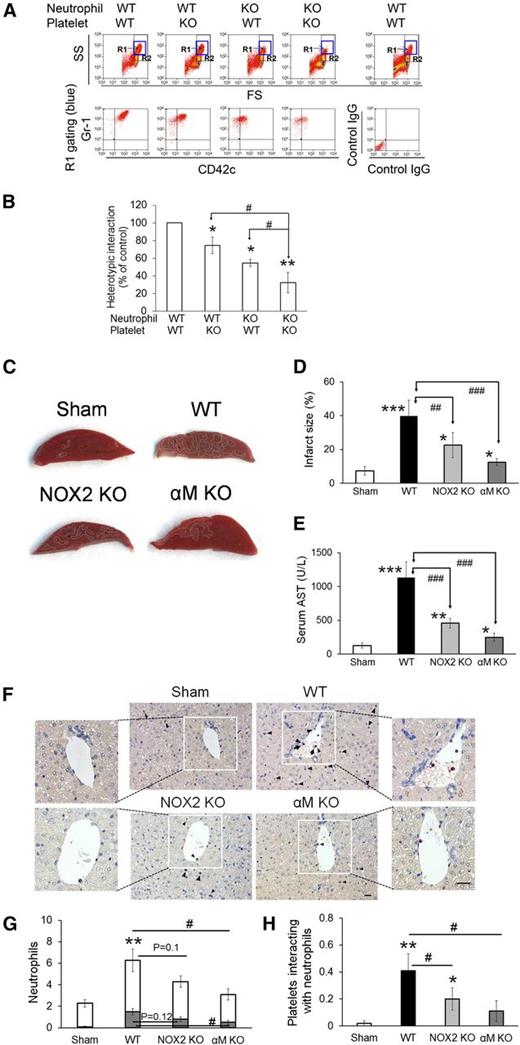
To investigate the role of NOX2 in regulating neutrophil-platelet interactions during vascular inflammation, we performed intravital microscopy in a mouse model of TNF-α-induced cremaster venular inflammation.1 Platelets and neutrophils were visualized by infusion of Dylight 488-conjugated anti-CD42c and Alexa Fluor 647-conjugated anti-Gr-1 antibodies, respectively. Because platelets mainly adhered to the top of adherent neutrophils, the fluorescence intensities of anti-CD42c antibodies were normalized to the number of adherent neutrophils and the length of vessels (Figure 1A). Compared with WT mice, NOX2 KO mice showed no defects in neutrophil rolling and adhesion to the TNF-α-inflamed endothelium, but exhibited nearly complete inhibition of neutrophil-platelet interactions on the inflamed venules (Figure 1B-E and supplemental Videos 1 and 2). Consistent with recent studies,14 there were no differences in the number of circulating blood cells between WT and NOX2 KO mice (Table 1). These results indicate that intravascular NOX2 is critical for neutrophil-platelet association during vascular inflammation.
Platelet and neutrophil NOX2 regulate heterotypic neutrophil-platelet interactions during TNF-α-induced venular inflammation. Intravital microscopy of WT and NOX2 KO mice was performed as described in the “Methods” section. Cremaster vascular inflammation was induced by intrascrotal injection of TNF-α. Three hours after TNF-α injection, neutrophils and platelets in inflamed venules were visualized by infusion of Alexa Fluor 647-conjugated anti-Gr-1 and Dylight 488-conjugated anti-CD42c antibodies, respectively, into the mice. (A) Representative images at various time points. Arrows show direction of blood flow. (B-C) Number of rolling and adherent neutrophils. (D-E) The integrated median fluorescence intensities of anti-CD42c antibodies (F platelets) were quantified, normalized by the number of adherent neutrophils and the vessel length, and plotted as a function of time. (E) F platelets in WT and NOX2 KO mice were compared at 60, 120, and 180 seconds after each capture (0 seconds). (F-G) WT and NOX2 KO platelets (F) or neutrophils (G) were isolated and labeled with calcein acetoxymethyl ester (calcein AM). The labeled cells (5 × 107 platelets or 106 neutrophils per mouse) were infused into TNF-α-inflamed mice. Endogenous neutrophils or platelets were visualized by infusion of Alexa Fluor 647-conjugated anti-Gr-1 or Dylight 649-conjugated anti-CD42c antibodies, respectively. Adhesion of the infused platelets to adherent neutrophils (F) and adhesion of endogenous platelets to infused neutrophils (G) were counted and normalized by the number of adherent neutrophils. Data represent the mean ± standard error of the mean (SEM) (n = 22-28 venules in 4 mice per group). *P < .05, **P < .01, or ***P < .001 vs WT control after Mann-Whitney test (E) or vs infusion of WT cells into WT mice (F-G) after Student t test. #P < .05 or ##P < .01 between two groups after Student t test.
Platelet and neutrophil NOX2 regulate heterotypic neutrophil-platelet interactions during TNF-α-induced venular inflammation. Intravital microscopy of WT and NOX2 KO mice was performed as described in the “Methods” section. Cremaster vascular inflammation was induced by intrascrotal injection of TNF-α. Three hours after TNF-α injection, neutrophils and platelets in inflamed venules were visualized by infusion of Alexa Fluor 647-conjugated anti-Gr-1 and Dylight 488-conjugated anti-CD42c antibodies, respectively, into the mice. (A) Representative images at various time points. Arrows show direction of blood flow. (B-C) Number of rolling and adherent neutrophils. (D-E) The integrated median fluorescence intensities of anti-CD42c antibodies (F platelets) were quantified, normalized by the number of adherent neutrophils and the vessel length, and plotted as a function of time. (E) F platelets in WT and NOX2 KO mice were compared at 60, 120, and 180 seconds after each capture (0 seconds). (F-G) WT and NOX2 KO platelets (F) or neutrophils (G) were isolated and labeled with calcein acetoxymethyl ester (calcein AM). The labeled cells (5 × 107 platelets or 106 neutrophils per mouse) were infused into TNF-α-inflamed mice. Endogenous neutrophils or platelets were visualized by infusion of Alexa Fluor 647-conjugated anti-Gr-1 or Dylight 649-conjugated anti-CD42c antibodies, respectively. Adhesion of the infused platelets to adherent neutrophils (F) and adhesion of endogenous platelets to infused neutrophils (G) were counted and normalized by the number of adherent neutrophils. Data represent the mean ± standard error of the mean (SEM) (n = 22-28 venules in 4 mice per group). *P < .05, **P < .01, or ***P < .001 vs WT control after Mann-Whitney test (E) or vs infusion of WT cells into WT mice (F-G) after Student t test. #P < .05 or ##P < .01 between two groups after Student t test.
To further examine the role of platelet and neutrophil NOX2 in the cell-cell interaction, we infused isolated, calcein acetoxymethyl ester (calcein AM)-labeled platelets or neutrophils into TNF-α-inflamed WT or NOX2 KO mice. Compared with WT platelets, infusion of NOX2 KO platelets into TNF-α-inflamed WT mice resulted in a significant decrease in adhesion of the infused platelets to adherent neutrophils, suggesting the important role of platelet NOX2 in mediating neutrophil-platelet interactions (Figure 1F). Adhesion of the infused platelets was further reduced when they were infused into TNF-α-inflamed NOX2 KO mice, implying the importance of neutrophil and/or nonhematopoietic cell NOX2 for neutrophil-platelet interactions. Conversely, compared with WT neutrophils, when NOX2 KO neutrophils were infused into TNF-α-inflamed WT mice, platelet adhesion to the infused neutrophils was significantly impaired (Figure 1G), suggesting that neutrophil NOX2 is important for neutrophil-platelet interactions. Infusion of the isolated WT or NOX2 KO neutrophils into TNF-α-inflamed NOX2 KO mice resulted in a further decrease in neutrophil-platelet interactions, compared with those observed in TNF-α-inflamed WT mice. These data suggest that NOX2 from both platelets and neutrophils plays important roles in mediating neutrophil-platelet interactions during vascular inflammation.
Although anti-NOX1 antibodies detected several proteins as a result of nonspecificity, the expression of NOX1 at 55 to 65 kDa was not altered in NOX2 KO platelets and neutrophils (supplemental Figure 1). Furthermore, WT and NOX2 KO ECs expressed a similar level of NOX4, a major NOX isoform in ECs,12 and no band was detected by control immunoglobulin G (data not shown). Thus, our results suggest that NOX1 and NOX4 are unlikely to compensate in NOX2 KO cells, which is in agreement with previous findings showing the unaltered expression of other NOXs in neutrophils from X-CGD patients.23
Hematopoietic and EC NOX2 are important for neutrophil-platelet interactions during venular inflammation
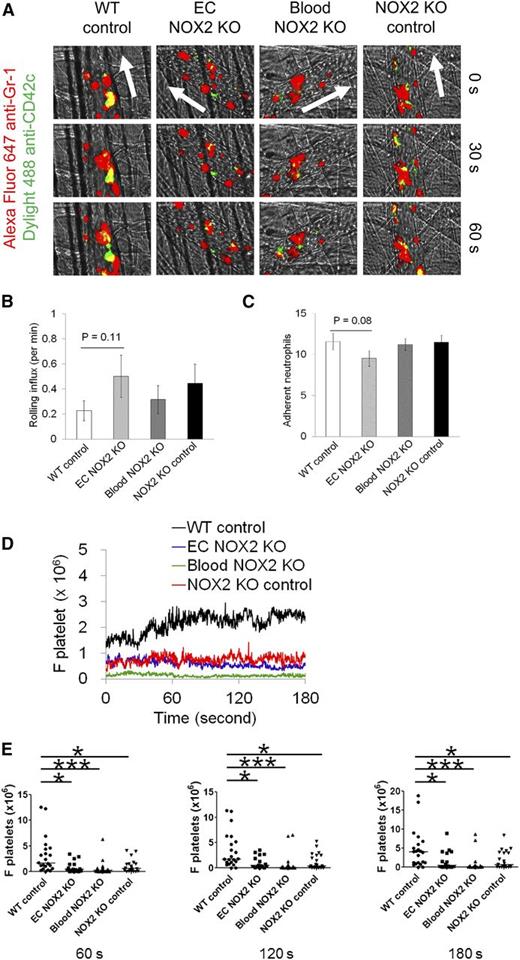
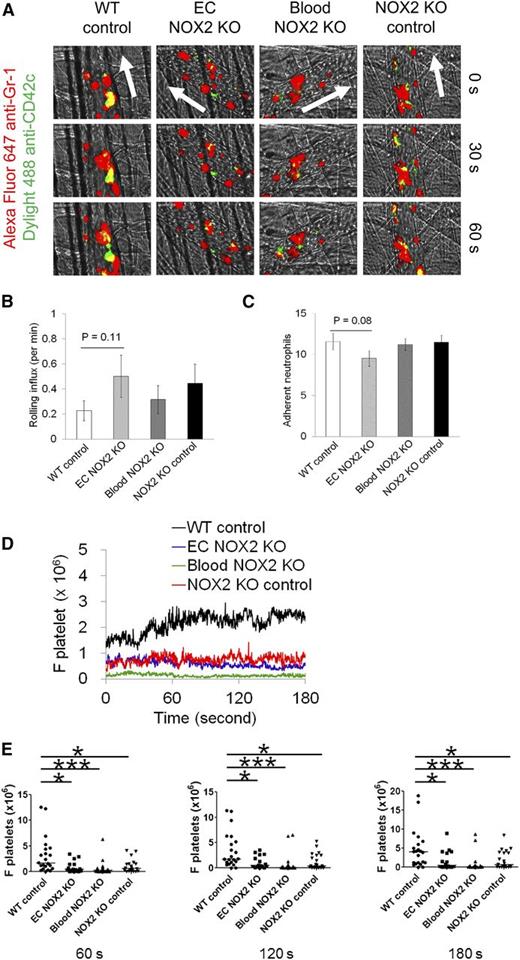
To dissect the role of hematopoietic and EC NOX2 in neutrophil-platelet interactions, the same intravital microscopy was carried out by using NOX2 bone marrow chimeric mice. The transplanted groups showed no significant differences in neutrophil rolling and adhesion during vascular inflammation (Figure 2A-C). However, neutrophil-platelet interactions were significantly reduced in EC NOX2, blood NOX2, and NOX2 KO control mice compared with WT control mice (Figure 2D-E). The number of circulating blood cells was similar in all groups (data not shown). These results suggest that both hematopoietic and EC NOX2 induce neutrophil-platelet interactions during venular inflammation. Although previous studies showed that TNF-α treatment induces NOX2 activation and ROS generation in cultured human pulmonary ECs,24 because of the experimental complexities in determining the role of EC NOX2 during neutrophil-platelet interactions, we focused on the role of platelet and neutrophil NOX2.
Hematopoietic and EC NOX2 are important for neutrophil-platelet interactions during venular inflammation. Intravital microscopy in NOX2 bone marrow chimeric mice was performed as described in Figure 1. (A) Representative images at various time points. Arrows show direction of blood flow. (B-C) Number of rolling and adherent neutrophils. (D-E) The fluorescence intensities of anti-CD42c antibodies (F platelets) and the comparison of F platelets among groups. Data represent the mean ± SEM (n = 22-24 venules in 4 mice per group). *P < .05 or ***P < .001 vs WT control after Mann-Whitney test.
Hematopoietic and EC NOX2 are important for neutrophil-platelet interactions during venular inflammation. Intravital microscopy in NOX2 bone marrow chimeric mice was performed as described in Figure 1. (A) Representative images at various time points. Arrows show direction of blood flow. (B-C) Number of rolling and adherent neutrophils. (D-E) The fluorescence intensities of anti-CD42c antibodies (F platelets) and the comparison of F platelets among groups. Data represent the mean ± SEM (n = 22-24 venules in 4 mice per group). *P < .05 or ***P < .001 vs WT control after Mann-Whitney test.
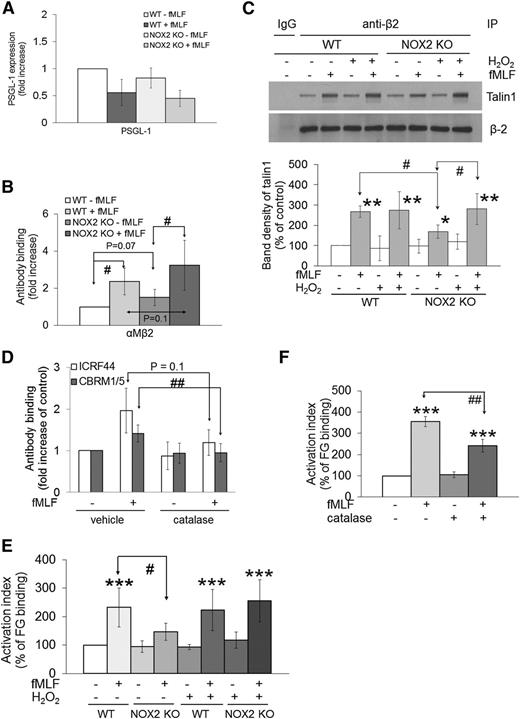
Platelet NOX2 regulates P-selectin exposure upon agonist stimulation and the ligand-binding function of GPIbα
Recent studies showed that NOX2 deletion did not affect P-selectin exposure when mouse platelets were activated with collagen-related peptide, a GPVI-specific agonist.14 We found that the surface expression of GPVI and GPIbα was also similar in WT and NOX2 KO platelets (supplemental Figure 2A-B). In addition, we observed that NOX2 deletion significantly decreased the surface expression of P-selectin and αIIbβ3 integrin in response to 0.025 but not 0.05 U/mL thrombin (Figure 3A and supplemental Figure 2C), suggesting that other signaling pathways induced by the increased concentration of thrombin compensate for the defective function of the KO platelets. Treatment of human platelets with catalase, a specific H2O2 scavenger, and diphenyleneiodonium (DPI), a nonspecific NOX inhibitor,16,25 significantly inhibited P-selectin exposure in response to 0.025 U/mL thrombin (Figure 3B). In control experiments, we found that NOX2 deletion or catalase treatment reduced intracellular ROS generation in both resting and activated mouse platelets and that compared with NOX2 deletion, treatment of NOX2 KO platelets with catalase further decreased ROS production (supplemental Figure 3A), implying that other pathways may also generate ROS. Consistent with previous studies,13 extracellular ROS were not detected in activated platelets (data not shown). These results suggest that platelet NOX2-generated ROS such as H2O2 regulate P-selectin exposure induced by submaximal concentrations of thrombin.
ROS produced from platelet NOX2 regulate P-selectin exposure and GPIbα function. (A) Mouse or (B) human platelets were pretreated with vehicle, catalase (1000 U/mL), dimethylsulfoxide (0.1%), or DPI (10 µM), and incubated with or without thrombin (0.025 U/mL in [B]). The surface expression of P-selectin (CD62P) was measured by flow cytometry. (C) Human platelets were treated with 1 μg/mL mouse immunoglobulin G1 (IgG1) or an anti-GPIbα antibody (VM16d), vehicle, or catalase followed by incubation with 10 μg/mL αMβ2 in the presence or absence of 0.5 mM MnCl2. The bound αMβ2 was determined by flow cytometry using goat anti-human β2 antibodies. (D) αMβ2 binding was measured in WT and NOX2 KO platelets as described in (C). (E-F) Mouse platelets were pretreated with or without catalase or 1 μM H2O2 and then incubated with 10 µg/mL vWF and 10 mM EDTA in the presence or absence of 10 µg/mL botrocetin. vWF binding was analyzed by flow cytometry using anti-vWF antibodies. Flow cytometric data are shown as the geometric mean fluorescence intensity (MFI) value or fold increase obtained by normalization of the MFI of antibodies to that of control IgG. (G) Mouse platelets were incubated with 10 µg/mL vWF and 10 µg/mL botrocetin. In some experiments, NOX2 KO platelets were pretreated with 0.1 to 1 μM H2O2. Platelet agglutination was measured in an aggregometer. All data represent the mean ± standard deviation (SD) (n = 4-5). *P < .05, **P < .01, or ***P < .001 between two groups after Student t test.
ROS produced from platelet NOX2 regulate P-selectin exposure and GPIbα function. (A) Mouse or (B) human platelets were pretreated with vehicle, catalase (1000 U/mL), dimethylsulfoxide (0.1%), or DPI (10 µM), and incubated with or without thrombin (0.025 U/mL in [B]). The surface expression of P-selectin (CD62P) was measured by flow cytometry. (C) Human platelets were treated with 1 μg/mL mouse immunoglobulin G1 (IgG1) or an anti-GPIbα antibody (VM16d), vehicle, or catalase followed by incubation with 10 μg/mL αMβ2 in the presence or absence of 0.5 mM MnCl2. The bound αMβ2 was determined by flow cytometry using goat anti-human β2 antibodies. (D) αMβ2 binding was measured in WT and NOX2 KO platelets as described in (C). (E-F) Mouse platelets were pretreated with or without catalase or 1 μM H2O2 and then incubated with 10 µg/mL vWF and 10 mM EDTA in the presence or absence of 10 µg/mL botrocetin. vWF binding was analyzed by flow cytometry using anti-vWF antibodies. Flow cytometric data are shown as the geometric mean fluorescence intensity (MFI) value or fold increase obtained by normalization of the MFI of antibodies to that of control IgG. (G) Mouse platelets were incubated with 10 µg/mL vWF and 10 µg/mL botrocetin. In some experiments, NOX2 KO platelets were pretreated with 0.1 to 1 μM H2O2. Platelet agglutination was measured in an aggregometer. All data represent the mean ± standard deviation (SD) (n = 4-5). *P < .05, **P < .01, or ***P < .001 between two groups after Student t test.
Because platelet GPIbα is important for platelet-neutrophil interactions,4 we examined whether its ligand-binding function is regulated by NOX2-generated ROS. We found that binding of soluble αMβ2 to resting human platelets was significantly increased in the presence of MnCl2, which activates the integrin and that the binding was significantly inhibited by catalase or VM16d, an anti-human GPIbα antibody blocking αMβ2-GPIbα binding (Figure 3C).7 In addition, although there are no available anti-mouse GPIbα antibodies that inhibit αMβ2-GPIbα binding, NOX2 deletion significantly impaired αMβ2 binding to mouse platelets in the presence of MnCl2 (Figure 3D). These results suggest that basal levels of ROS including H2O2, which are generated by NOX2 probably as a result of spontaneous activation of isolated platelets, are important for binding of activated αMβ2 to GPIbα. We also observed that binding of vWF to platelet GPIbα was significantly decreased in NOX2 KO platelets compared with WT platelets (Figure 3E). Furthermore, catalase treatment significantly inhibited vWF binding to WT but not to NOX2 KO platelets. Interestingly, treatment with 1 µM H2O2 rescued the defect in vWF binding to the KO platelets (Figure 3F). Consistent with these binding studies, platelet agglutination mediated by the vWF-GPIbα interaction was also markedly inhibited by NOX2 deletion, and the defect was rescued when the KO platelets were pretreated with 1 µM H2O2 (Figure 3G). However, 1 µM H2O2 did not affect agglutination of WT platelets (data not shown). Taken together, these results suggest that NOX2-generated H2O2 regulates the ligand-binding function of GPIbα.
Neutrophil NOX2 is required for the activation and ligand-binding activity of αMβ2 integrin during cell activation
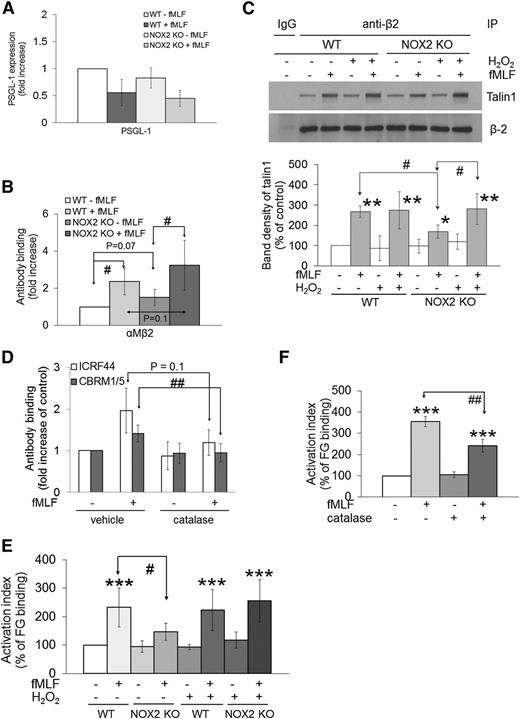
Neutrophil PSGL-1 and αMβ2 integrin are two major receptors necessary for the interaction with platelets.2 We observed that WT and NOX2 KO neutrophils showed similar expression levels of PSGL-1 and αMβ2 integrin before and after fMLF stimulation (Figure 4A-B). To investigate whether NOX2 regulates αMβ2 integrin activation, we examined β2-talin1 binding, a final step of integrin activation.1 As determined by co-immunoprecipitation assays, β2-talin1 binding was significantly reduced in fMLF-stimulated NOX2 KO neutrophils compared with WT neutrophils (Figure 4C). Treatment with 1 μM H2O2 did not affect β2-talin1 binding in WT neutrophils but restored the decreased binding in NOX2 KO cells, supporting a role for NOX2-generated H2O2 in β2 integrin activation.
ROS generated from neutrophil NOX2 are crucial for the activation and ligand-binding activity of αMβ2 integrin. (A-B) Mouse neutrophils were treated with or without fMLF, and the surface expression of PSGL-1 and αMβ2 integrin was measured by flow cytometry. (C) WT and NOX2 KO neutrophils were pretreated with or without 1 μM H2O2 and stimulated with fMLF. Lysates were immunoprecipitated (IP) with control IgG or anti-β2 antibodies, followed by immunoblotting and densitometry. (D) Human neutrophils pretreated with 1000 U/mL catalase were stimulated with fMLF, followed by flow cytometry using antibodies against total (ICRF44) and activated αMβ2 (CBRM1/5). Data are shown as a fold increase obtained by normalization of the geometric MFI value of antibodies to that of control IgG. (E-F) Mouse (E) or human (F) neutrophils were pretreated with or without H2O2 (1 µM) or catalase and then incubated with Alexa Fluor 488-conjugated FG in the absence or presence of fMLF. Untreated cells are shown as 100% (white bar). Data represent the mean ± SD (n = 3-4). *P < .05, **P < .01, or ***P < .001 vs WT or vehicle control after analysis of variance (ANOVA) and Dunnett’s test, and #P < .05 or ##P < .01 vs WT or vehicle control after Student t test.
ROS generated from neutrophil NOX2 are crucial for the activation and ligand-binding activity of αMβ2 integrin. (A-B) Mouse neutrophils were treated with or without fMLF, and the surface expression of PSGL-1 and αMβ2 integrin was measured by flow cytometry. (C) WT and NOX2 KO neutrophils were pretreated with or without 1 μM H2O2 and stimulated with fMLF. Lysates were immunoprecipitated (IP) with control IgG or anti-β2 antibodies, followed by immunoblotting and densitometry. (D) Human neutrophils pretreated with 1000 U/mL catalase were stimulated with fMLF, followed by flow cytometry using antibodies against total (ICRF44) and activated αMβ2 (CBRM1/5). Data are shown as a fold increase obtained by normalization of the geometric MFI value of antibodies to that of control IgG. (E-F) Mouse (E) or human (F) neutrophils were pretreated with or without H2O2 (1 µM) or catalase and then incubated with Alexa Fluor 488-conjugated FG in the absence or presence of fMLF. Untreated cells are shown as 100% (white bar). Data represent the mean ± SD (n = 3-4). *P < .05, **P < .01, or ***P < .001 vs WT or vehicle control after analysis of variance (ANOVA) and Dunnett’s test, and #P < .05 or ##P < .01 vs WT or vehicle control after Student t test.
By using specific antibodies to total (ICRF44) and activated (CBRM1/5) human αMβ2 integrin, we also observed that the expression and activation of αMβ2 integrin increased upon fMLF stimulation and that catalase treatment significantly inhibited binding of CBRM1/5, but not ICRF44, to fMLF-stimulated neutrophils (Figure 4D). In control experiments, NOX2 deletion or treatment of human neutrophils with DPI resulted in a remarkable reduction of extracellular and intracellular ROS generation during neutrophil activation as measured by the signal of 2′,7′-dichlorofluorescein diacetate (DCFH-DA), lucigenin, and Amplex Red (supplemental Figure 3B-F). Catalase treatment inhibited generation of H2O2 but not superoxide anions in fMLF-stimulated human neutrophils (supplemental Figure 3D,F). These data suggest that NOX2-generated ROS, mainly H2O2, regulate αMβ2 integrin activation without affecting its membrane translocation following fMLF stimulation.
By using Alexa Fluor 488-conjugated FG, a ligand for αMβ2 integrin, we observed that FG binding was significantly reduced in fMLF-stimulated NOX2 KO neutrophils compared with WT neutrophils (Figure 4E). Pretreatment with 1 μM H2O2 rescued the defect in the KO neutrophils without altering FG binding to WT cells. Moreover, FG binding was significantly decreased when human neutrophils were pretreated with catalase (Figure 4F). Taken together, our results indicate that neutrophil NOX2-produced H2O2 is important for the activation and ligand-binding activity of αMβ2 integrin during cell activation.
NOX2 is important for neutrophil-platelet aggregation under stirring conditions and for hepatic ischemia/reperfusion injury in mice
We and others showed that neutrophils aggregate with activated platelets under stirring conditions.1,26 By using this in vitro assay, we sought to determine the role of NOX2 in neutrophil-platelet aggregation. It was reported that treatment of suspended neutrophils with TNF-α does not induce NOX2-mediated ROS generation but primes the cells, thereby enhancing their response to additional agonists.27 Thus, to mimic the in vivo inflammatory conditions, neutrophils were labeled with Alexa Fluor 647-conjugated anti-Gr-1 antibodies and treated with TNF-α. Platelets were labeled with Dylight 488-conjugated anti-CD42c antibodies, activated with 0.025 U/mL thrombin, and mixed with TNF-α-treated neutrophils under stirring conditions. Consistent with our previous report,1 a new cell population (R1 gate) of neutrophil-platelet aggregates appeared above the neutrophil population, and most cells in the R1 gate were positive for both Gr-1 and CD42c (Figure 5A). A mixture of unstimulated platelets and neutrophils did not form cell-cell aggregates (data not shown), suggesting that P-selectin on activated platelets is required to initiate the interaction with neutrophils in this assay. As quantified by the fluorescence intensity of the anti-CD42c antibody, NOX2 deletion in either platelets or neutrophils moderately inhibited neutrophil-platelet aggregation (Figure 5B). When NOX2 was deleted in both cell types, the inhibitory effects were further potentiated. These results suggest that both platelet and neutrophil NOX2 regulate neutrophil-platelet aggregation under shear.
NOX2 is important for neutrophil-platelet aggregation under stirring conditions and for hepatic ischemia/reperfusion injury in vivo. (A-B) In vitro neutrophil-platelet aggregation assay was performed as described in the “Methods” section. Neutrophils and platelets isolated from WT and NOX2 KO mice were labeled with Alexa Fluor 647-conjugated anti-Gr-1 and Dylight 488-conjugated anti-CD42c antibodies, respectively. Thrombin-activated platelets were mixed with TNF-α-treated neutrophils under stirring conditions (1000 rpm). Cells were analyzed by flow cytometry. R1 designates leukocyte-platelet aggregates and R2 designates total population of neutrophils. Neutrophil-platelet aggregation was measured by the fluorescence intensity of anti-CD42c antibodies (B) in the R1 gate. Data represent the mean ± SD (n = 3). SS, side scatter; FS, forward scatter. (C-F) Hepatic ischemia/reperfusion injury was induced as described in the “Methods” section. (C) Representative pictures of liver sections stained with triphenyltetrazolium chloride. (D) Infarct sizes (white areas in C) were measured by Image J. (E) Serum levels of aspartate aminotransferase (AST). (F) Neutrophils (arrow heads) and platelets (arrows) in liver sections were stained with an esterase kit (pink) and anti-CD41 antibodies (brown), respectively. Nucleated cells were stained with hematoxylin (purple). Bar = 20 μm. (G) The number of neutrophils was counted inside (gray) and outside (white) the hepatic vessels. (H) The number of platelets that interact with adherent neutrophils to the vessels was counted. Data represent the mean ± SD (n = 5 mice and 15 sections in 5 mice for G-H). *P < .05, **P < .01, or ***P < .001 vs WT control or sham after ANOVA and Dunnett’s test. In (G), **P < .01 was obtained after comparison of the total number of neutrophils. #P < .05, ##P < .01, and ###P < .001 between 2 groups after Student t test.
NOX2 is important for neutrophil-platelet aggregation under stirring conditions and for hepatic ischemia/reperfusion injury in vivo. (A-B) In vitro neutrophil-platelet aggregation assay was performed as described in the “Methods” section. Neutrophils and platelets isolated from WT and NOX2 KO mice were labeled with Alexa Fluor 647-conjugated anti-Gr-1 and Dylight 488-conjugated anti-CD42c antibodies, respectively. Thrombin-activated platelets were mixed with TNF-α-treated neutrophils under stirring conditions (1000 rpm). Cells were analyzed by flow cytometry. R1 designates leukocyte-platelet aggregates and R2 designates total population of neutrophils. Neutrophil-platelet aggregation was measured by the fluorescence intensity of anti-CD42c antibodies (B) in the R1 gate. Data represent the mean ± SD (n = 3). SS, side scatter; FS, forward scatter. (C-F) Hepatic ischemia/reperfusion injury was induced as described in the “Methods” section. (C) Representative pictures of liver sections stained with triphenyltetrazolium chloride. (D) Infarct sizes (white areas in C) were measured by Image J. (E) Serum levels of aspartate aminotransferase (AST). (F) Neutrophils (arrow heads) and platelets (arrows) in liver sections were stained with an esterase kit (pink) and anti-CD41 antibodies (brown), respectively. Nucleated cells were stained with hematoxylin (purple). Bar = 20 μm. (G) The number of neutrophils was counted inside (gray) and outside (white) the hepatic vessels. (H) The number of platelets that interact with adherent neutrophils to the vessels was counted. Data represent the mean ± SD (n = 5 mice and 15 sections in 5 mice for G-H). *P < .05, **P < .01, or ***P < .001 vs WT control or sham after ANOVA and Dunnett’s test. In (G), **P < .01 was obtained after comparison of the total number of neutrophils. #P < .05, ##P < .01, and ###P < .001 between 2 groups after Student t test.
To further explore the role of NOX2-mediated neutrophil-platelet interactions in thromboinflammatory disease, we used a mouse model of hepatic ischemia/reperfusion injury. Previous studies showed that not only platelets and neutrophils but also ROS are important for the pathogenesis of hepatic ischemia/reperfusion injury.28,29 We found that compared with WT mice, NOX2 KO mice exhibited a significant reduction in the infarct size and serum levels of aspartate aminotransferase (AST) (Figure 5C-E). Furthermore, deletion of αMβ2 integrin, a critical receptor for neutrophil-platelet interactions and neutrophil transmigration, resulted in a remarkable decrease in the infarct size and AST levels. As observed by histochemistry, the number of neutrophils within the hepatic vessels and the surrounding interstitium was moderately but not significantly (P = .12 and P = .1, respectively) reduced in NOX2 KO mice compared with WT mice (Figure 5F-G). When we further measured the number of platelets that interact with neutrophils adherent to the vessels, platelet-neutrophil interactions were significantly reduced in NOX2 KO mice, compared with WT mice (Figure 5H). Deletion of αM resulted in a significant decrease in adhesion and transmigration of neutrophils and platelet-neutrophil interactions in the hepatic vessels. These results suggest that NOX2-mediated neutrophil-platelet interactions may play an important role during the pathophysiology of hepatic ischemia/reperfusion injury.
Platelet and neutrophil NOX2 differentially modulate Ca2+ mobilization
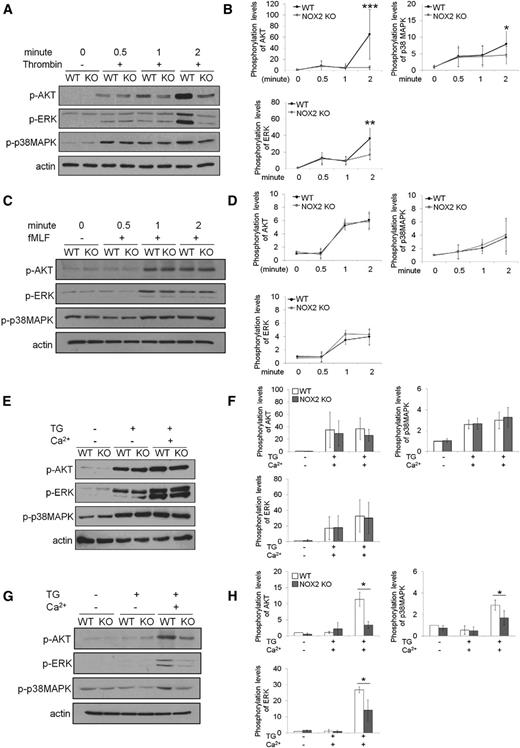
Recent studies showed that neutrophil NOX2 activation requires store-operated Ca2+ entry (SOCE).30 However, it remains poorly understood whether NOX2 deletion affects Ca2+ mobilization, which is critical for regulating the function of cell surface receptors.31 We found that compared with WT platelets, NOX2 KO platelets significantly inhibited intracellular Ca2+ release upon thrombin stimulation (Figure 6A) but did not alter SOCE in the presence of 2 mM CaCl2. Consistently, NOX2 deletion did not affect SOCE following Ca2+ depletion induced by thapsigargin (TG), an inhibitor of sarco/endoplasmic reticulum (ER) Ca2+-ATPase (Figure 6B). In contrast, fMLF-stimulated WT and NOX2 KO neutrophils released similar levels of Ca2+ from the ER (Figure 6C). However, SOCE induced by fMLF or TG was significantly reduced in NOX2 KO neutrophils compared with WT cells (Figure 6C-D). The expression levels of stromal interaction molecule 1 (STIM1), a Ca2+ sensor essential for SOCE, and ORAI1, a plasma membrane Ca2+ channel,32 were similar between WT and NOX2 KO cells (data not shown). These results indicate that platelet NOX2 regulates intracellular Ca2+ release but not SOCE, whereas neutrophil NOX2 is important for SOCE but not ER Ca2+ release during cell activation.
Platelet and neutrophil NOX2 differentially regulate Ca2+ release and influx. Mouse platelets (A-B) and neutrophils (C-D) were pretreated with Ca2+ dye and then incubated with thrombin (0.025 U/mL [A]), fMLF (C), or thapsigargin (200 nM [B,D]) for 3-4 minutes and 2 mM CaCl2 was then added. Intracellular Ca2+ release and SOCE were measured and quantified by the AUC (arbitrary units). Quantitative data represent the mean ± SD (n = 4-5). *P < .05 or **P < .01 vs WT after Student t test.
Platelet and neutrophil NOX2 differentially regulate Ca2+ release and influx. Mouse platelets (A-B) and neutrophils (C-D) were pretreated with Ca2+ dye and then incubated with thrombin (0.025 U/mL [A]), fMLF (C), or thapsigargin (200 nM [B,D]) for 3-4 minutes and 2 mM CaCl2 was then added. Intracellular Ca2+ release and SOCE were measured and quantified by the AUC (arbitrary units). Quantitative data represent the mean ± SD (n = 4-5). *P < .05 or **P < .01 vs WT after Student t test.
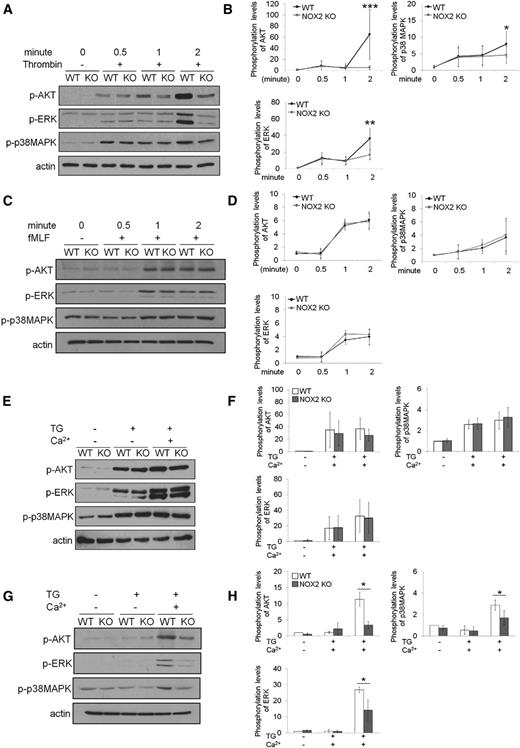
It was reported that platelet AKT signaling elicited by the binding of agonists to G-protein-coupled receptors is crucial for Ca2+ signaling33 and that neutrophil AKT2 is critical for Ca2+ mobilization during cell activation.1 To explain the differential regulation of Ca2+ signaling in platelets and neutrophils by NOX2, we examined the phosphorylation of AKT and MAP kinases ERK and p38. Compared with WT platelets, NOX2 KO platelets exhibited a significant decrease in the phosphorylation of AKT, ERK, and p38MAPK 2 minutes after thrombin stimulation (Figure 7A-B). However, no difference was observed in the phosphorylation of AKT, ERK, and p38MAPK in fMLF-stimulated WT and NOX2 KO neutrophils (Figure 7C-D). Because it was difficult to compare different agonist-induced signaling pathways in different cell types, we further examined how phosphorylation of those kinases is affected during TG-induced SOCE. We found that WT and NOX2 KO platelets showed similar levels of AKT, ERK, and p38MAPK phosphorylation during TG-induced Ca2+ depletion and SOCE (Figure 7E-F). In contrast, the phosphorylation levels of those kinases were significantly reduced during SOCE, but not TG-induced Ca2+ depletion, in NOX2 KO neutrophils compared with WT cells (Figure 7G-H). Treatment with 1 μM H2O2 restored the decreased phosphorylation of those kinases in NOX2 KO neutrophils (supplemental Figure 4), suggesting the role of NOX2-produced H2O2 in regulating activity of protein kinases. Taken together, our results show that the differential regulation of Ca2+ mobilization by platelet and neutrophil NOX2 correlates with the differences in the phosphorylation of AKT, ERK, and p38MAPK.
Platelet and neutrophil NOX2 are important for regulating the phosphorylation of AKT, ERK, and p38MAPK. (A-B) Mouse platelets and (C-D) neutrophils were treated with thrombin (0.025 U/mL) and fMLF, respectively, for 0.5, 1, and 2 minutes. (E-F) Platelets and (G-H) neutrophils were treated with 200 nM TG for 3 minutes, and 2 mM CaCl2 was then added and incubated for 1 minute. Cells were lysed at different time points, followed by immunoblotting with equal amounts of proteins and densitometry. (A,C,E,G) Representative blots. (B,D,F,H) Quantitative graphs. Data represent the mean ± SD (n = 4-6). *P < .05, **P < .01, or ***P < .001 vs WT after Student t test.
Platelet and neutrophil NOX2 are important for regulating the phosphorylation of AKT, ERK, and p38MAPK. (A-B) Mouse platelets and (C-D) neutrophils were treated with thrombin (0.025 U/mL) and fMLF, respectively, for 0.5, 1, and 2 minutes. (E-F) Platelets and (G-H) neutrophils were treated with 200 nM TG for 3 minutes, and 2 mM CaCl2 was then added and incubated for 1 minute. Cells were lysed at different time points, followed by immunoblotting with equal amounts of proteins and densitometry. (A,C,E,G) Representative blots. (B,D,F,H) Quantitative graphs. Data represent the mean ± SD (n = 4-6). *P < .05, **P < .01, or ***P < .001 vs WT after Student t test.
Discussion
This study demonstrates that NOX2 from both platelets and neutrophils plays critical roles in regulating the function of surface receptors, including GPIbα and αMβ2 integrin, which are essential for neutrophil-platelet interactions during vascular inflammation. Previous studies suggested that activation of both platelets and leukocytes needs to be controlled to treat thromboinflammatory conditions such as hepatic and cerebral ischemia/reperfusion injury28,29,34-36 and that inhibition or deletion of GPIbα and αMβ2 integrin, but not αIIbβ3, protects mice following ischemia/reperfusion injury in a middle cerebral artery.34,36,37 Although there are some reports showing the role of NOX1 and NOX4 in stroke,38,39 several studies using pharmacologic and genetic approaches demonstrated that NOX2 is crucial for the pathogenesis of ischemic stroke40,41 and that hematopoietic cell NOX2 contributes more to the development of stroke than endothelial or brain resident cell NOX2.42 Because neutrophil-platelet interactions are important for vascular occlusion under thromboinflammatory conditions1 and because intravascular cell NOX2 regulates neutrophil-platelet interactions during vascular inflammation (Figures 1 and 2), our findings in part explain how NOX2 KO mice show protective effects in ischemic stroke. Furthermore, our in vivo studies implicate the importance of NOX2-mediated platelet-neutrophil interactions during hepatic ischemia/reperfusion injury. Although future studies are required to explore whether our findings in mice are observed in X-CGD patients and to investigate the role of other NOX isoforms in neutrophil-platelet interactions, our results together with previous findings40,41 support NOX2 as a potential therapeutic target for the treatment of vascular disease.
NOX2 is mainly localized on the plasma membrane of all intravascular cells, and the activated NOX2 complex produces extracellular superoxide anions.12 Our studies revealed that NOX2 deletion or treatment with catalase or DPI results in a significant decrease in P-selectin exposure on thrombin-activated platelets, suggesting that ROS produced by platelet NOX2 could be important under certain disease conditions. Although platelets may not be fully activated by granular molecules and cytokines released from activated neutrophils and ECs during vascular inflammation because those agonists are unlikely to be as potent as thrombin or collagen, our intravital microscopic studies clearly demonstrate that platelet NOX2 is important for regulating neutrophil-platelet interactions during vascular inflammation (Figure 1F). Moreover, our in vitro assay showed that deletion of platelet NOX2 significantly reduces neutrophil-platelet aggregation under stirring conditions, proving the importance of platelet NOX2 for neutrophil-platelet interactions.
Previous studies demonstrated that binding of αMβ2 integrin to GPIbα of the GPIb-IX-V complex mediates stable attachment between neutrophils and platelets and that the binding sites of vWF and αMβ2 integrin on GPIbα may partially overlap.7 Although platelets express other binding molecules for αMβ2, such as junctional adhesion molecule-3,43 our studies using VM16d and catalase showed the importance of platelet-derived H2O2 in regulating binding of αMβ2 to GPIbα. Moreover, we found that deletion of platelet NOX2 or inhibition of H2O2 impairs binding of vWF to GPIbα. These results suggest that NOX2-generated ROS are important for regulating the ligand-binding function of GPIbα. Although it remains poorly understood how GPIbα function is regulated under oxidative stress, our results imply that ROS-mediated oxidation of sulfhydryl groups in GPIbα may alter its ligand-binding affinity.
The ligand-binding activity of αMβ2 integrin is affected by its membrane redistribution, conformational change, and clustering during neutrophil activation.44,45 We reported that neutrophil AKT2 controls intracellular Ca2+ release and the membrane translocation and activation of αMβ2 integrin during cell activation.1 Furthermore, previous studies showed that neutrophil AKT2 phosphorylates p47phox and thus activates NOX2.9 We found that NOX2-produced ROS are important for activation but not membrane translocation of αMβ2 integrin, thereby affecting the ligand-binding activity (Figure 4). We consistently observed that a combination of NOX2 KO neutrophils with blocking anti-αM antibodies does not potentiate the inhibitory effect on neutrophil-platelet aggregation in vitro (supplemental Figure 5). The differential regulation between AKT2 and NOX2 could result from additional effects of AKT2, such as intracellular Ca2+ release and activation of other protein kinases, which may influence exocytosis-mediated membrane translocation of αMβ2 integrin.1 Previously, we demonstrated that neutrophil surface protein disulfide isomerase (PDI), a cell-surface localized thiol isomerase, plays a fine-tuning role in regulating the clustering and ligand-binding activity of αMβ2 integrin after agonist stimulation.22 Since the isomerase activity of PDI is controlled in a redox-sensitive manner,46 NOX2-produced ROS may alter PDI activity. Therefore, our results suggest that the ligand-binding activity of αMβ2 integrin could be differentially and/or sequentially modulated by several different molecules under inflammatory conditions. In addition, extracellular ROS produced from activated ECs may influence the redox state of αMβ2 integrin and thus regulate neutrophil-platelet interactions during vascular inflammation. It is of importance to note that intravascular cell NOX2 is not critical for neutrophil rolling and adhesion to activated ECs but controls neutrophil-platelet interactions during vascular inflammation, suggesting that the regulatory effect of NOX2 would not be universal but specific to the molecules whose functions are regulated under oxidative stress.
We demonstrated that platelet and neutrophil NOX2 specifically regulate intracellular Ca2+ release and SOCE, respectively, during cell activation, which correlates with the phosphorylation of AKT, ERK, and p38MAPK. Recent studies revealed that neutrophil NOX2 is important for the activities of phosphatase and tensin homolog (PTEN) and AKT47 and that SOCE activates PKCα/β, thereby phosphorylating p47phox during neutrophil activation.30 However, we found that PTEN activity is reduced in both NOX2 KO platelets and neutrophils after agonist stimulation (supplemental Figure 6) and that there is no difference in PKCα/β phosphorylation between WT and NOX2 KO cells after agonist stimulation or TG-induced SOCE (supplemental Figure 7). Thus, consistent with our recent report demonstrating the importance of AKT2 in Ca2+ signaling during neutrophil activation,1 these findings provide evidence that AKT signaling regulates ROS-mediated Ca2+ signaling. We speculate that intracellular Ca2+ release modulates an early stage of cell activation such as granular secretion, whereas SOCE is involved in a later stage of cell activation such as integrin activation. Although the detailed mechanisms remain to be investigated, our results suggest that Ca2+ signaling and kinase activity are mutually regulated. Previous studies showed that treatment of healthy neutrophils with fMLF following TG-induced Ca2+ depletion rapidly inhibits Ca2+ influx resulting from plasma membrane depolarization mediated by NOX2-generated superoxide anions and that such inhibition in Ca2+ influx is not seen in the neutrophils of X-CGD patients.48 We consistently found that compared with WT neutrophils, NOX2 KO neutrophils show a smaller decrease in Ca2+ influx after fMLF stimulation during TG-induced SOCE (supplemental Figure 8). It will be interesting to test whether Ca2+ mobilization is differentially regulated in the platelets and neutrophils of X-CGD patients.
Overall, our studies provide mechanistic insight into how platelet and neutrophil NOX2 regulate the function of surface receptors, thereby playing an important role in neutrophil-platelet interactions during vascular inflammation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grant R01 HL109439 from the National Institutes of Health, National Heart, Lung, and Blood Institute. J.L. and K.K. are recipients of American Heart Association postdoctoral fellowships.
Authorship
Contribution: K.K. and J.L. designed and performed research, collected and analyzed data, and wrote the manuscript; A.T. performed research and wrote the manuscript; R.K.A. provided important reagents; and J.C. designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jaehyung Cho, 835 S. Wolcott Ave, E403, Chicago, IL 60612; e-mail: thromres@uic.edu.
References
Author notes
K.K. and J.L. contributed equally to this study.

![Figure 3. ROS produced from platelet NOX2 regulate P-selectin exposure and GPIbα function. (A) Mouse or (B) human platelets were pretreated with vehicle, catalase (1000 U/mL), dimethylsulfoxide (0.1%), or DPI (10 µM), and incubated with or without thrombin (0.025 U/mL in [B]). The surface expression of P-selectin (CD62P) was measured by flow cytometry. (C) Human platelets were treated with 1 μg/mL mouse immunoglobulin G1 (IgG1) or an anti-GPIbα antibody (VM16d), vehicle, or catalase followed by incubation with 10 μg/mL αMβ2 in the presence or absence of 0.5 mM MnCl2. The bound αMβ2 was determined by flow cytometry using goat anti-human β2 antibodies. (D) αMβ2 binding was measured in WT and NOX2 KO platelets as described in (C). (E-F) Mouse platelets were pretreated with or without catalase or 1 μM H2O2 and then incubated with 10 µg/mL vWF and 10 mM EDTA in the presence or absence of 10 µg/mL botrocetin. vWF binding was analyzed by flow cytometry using anti-vWF antibodies. Flow cytometric data are shown as the geometric mean fluorescence intensity (MFI) value or fold increase obtained by normalization of the MFI of antibodies to that of control IgG. (G) Mouse platelets were incubated with 10 µg/mL vWF and 10 µg/mL botrocetin. In some experiments, NOX2 KO platelets were pretreated with 0.1 to 1 μM H2O2. Platelet agglutination was measured in an aggregometer. All data represent the mean ± standard deviation (SD) (n = 4-5). *P < .05, **P < .01, or ***P < .001 between two groups after Student t test.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/126/16/10.1182_blood-2014-10-605261/4/m_1952f3.jpeg?Expires=1766138637&Signature=xJRnh1GwjpHWdsg5spabRq1~nwFl2MRWqyICfGTV7o9~UclbxnZwW1X2EWMENzf30rqPWKsy52U4L80guzPMFy5Waz-byyIdstIMY8jb-rNZMA6Jsvt7x-ZPU~59P-2EFs7ijAeMxlXXMqn0bGCKTCyGm66l1~3TCCVwmx1SxXlkAiMhCsutARfzYtY3-~LHSzqeXJx3xwxJl~jMJGkV5y1ERDM0YMLKKx6zts5bUQmkdFdSrHqNFqfMNq1xJShBztZMqZi6l-v5bHdo5aQU8~WIWsgCStUs0I0rrUwlgJzgJHdRuSIyzFRnasLjZVeo2ADHKovwToIrcHdT87hbVA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Platelet and neutrophil NOX2 differentially regulate Ca2+ release and influx. Mouse platelets (A-B) and neutrophils (C-D) were pretreated with Ca2+ dye and then incubated with thrombin (0.025 U/mL [A]), fMLF (C), or thapsigargin (200 nM [B,D]) for 3-4 minutes and 2 mM CaCl2 was then added. Intracellular Ca2+ release and SOCE were measured and quantified by the AUC (arbitrary units). Quantitative data represent the mean ± SD (n = 4-5). *P < .05 or **P < .01 vs WT after Student t test.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/126/16/10.1182_blood-2014-10-605261/4/m_1952f6.jpeg?Expires=1766138637&Signature=XjABgacBuGrRxHOLDzjTl7iSsU7atcNR3emTt0x82FTQKCWIIkNRKVFV4aEcJr6l5l3nhZhtgEiQKHz29cQX2pGQ3IozLB16LeaqWD9cat26PzL~haWaCUvVhYzaSYvSxtQUlGRrOY1eRdB0GBdUAUVbzXEKpSPj0S4ApVC282W4BCLtL4PV2Owok9s~W7rzgVl-PoWu6Ui1w-HM4ovynEs69YIUgxcGCge7U2Oi2dnb6quoz8nNxmDsWYAtQ-Viiu79tdgqWEAM2HbqlESIal6eheS2TLOkRlRsEzYebonGt7x7NOIMnBvJapHzrVzhFMdpHfilhGMHuWmnXC1Sqw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 3. ROS produced from platelet NOX2 regulate P-selectin exposure and GPIbα function. (A) Mouse or (B) human platelets were pretreated with vehicle, catalase (1000 U/mL), dimethylsulfoxide (0.1%), or DPI (10 µM), and incubated with or without thrombin (0.025 U/mL in [B]). The surface expression of P-selectin (CD62P) was measured by flow cytometry. (C) Human platelets were treated with 1 μg/mL mouse immunoglobulin G1 (IgG1) or an anti-GPIbα antibody (VM16d), vehicle, or catalase followed by incubation with 10 μg/mL αMβ2 in the presence or absence of 0.5 mM MnCl2. The bound αMβ2 was determined by flow cytometry using goat anti-human β2 antibodies. (D) αMβ2 binding was measured in WT and NOX2 KO platelets as described in (C). (E-F) Mouse platelets were pretreated with or without catalase or 1 μM H2O2 and then incubated with 10 µg/mL vWF and 10 mM EDTA in the presence or absence of 10 µg/mL botrocetin. vWF binding was analyzed by flow cytometry using anti-vWF antibodies. Flow cytometric data are shown as the geometric mean fluorescence intensity (MFI) value or fold increase obtained by normalization of the MFI of antibodies to that of control IgG. (G) Mouse platelets were incubated with 10 µg/mL vWF and 10 µg/mL botrocetin. In some experiments, NOX2 KO platelets were pretreated with 0.1 to 1 μM H2O2. Platelet agglutination was measured in an aggregometer. All data represent the mean ± standard deviation (SD) (n = 4-5). *P < .05, **P < .01, or ***P < .001 between two groups after Student t test.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/126/16/10.1182_blood-2014-10-605261/4/m_1952f3.jpeg?Expires=1767229553&Signature=F3xFHBd~t0eeBAS2luUTLlzeBnKqeAya9-e0BpGmVzuWPlqOd7rdszDp~ziAlEVHG0S52yLNh-SWdWKhXaLeQ~WHfn9mejxxN1g7wY3Ys7JmqlYNdweKVJGu9ZAnsGGoVYcCAJijrkxtnh-uBeS4Uwbt4qQDw01A9Y0MTNDEEu8~oJcjKqMrYX2v9oyuHjmVvDxLFIQcBshwl-~cHkiFDRtrs4-2F5Bpa6dDnNBZ19CEKO6NaAbXfS7MsDvrrxAauq66sHWvfWE3q66rGHMa0T5CrPshwrXYz7nW58S5zv~0lHeGXws0c-UfKWoBU4xOqr5WPb6pltC12QPcTPpU0g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Platelet and neutrophil NOX2 differentially regulate Ca2+ release and influx. Mouse platelets (A-B) and neutrophils (C-D) were pretreated with Ca2+ dye and then incubated with thrombin (0.025 U/mL [A]), fMLF (C), or thapsigargin (200 nM [B,D]) for 3-4 minutes and 2 mM CaCl2 was then added. Intracellular Ca2+ release and SOCE were measured and quantified by the AUC (arbitrary units). Quantitative data represent the mean ± SD (n = 4-5). *P < .05 or **P < .01 vs WT after Student t test.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/126/16/10.1182_blood-2014-10-605261/4/m_1952f6.jpeg?Expires=1767229553&Signature=kCnl-9G8BJpfFhHQgZcGyvUtJyc722-bXGOJuh27aN383l4K0OzaIdWpnrtq199T2t5HVHLTzhh0R44EP5kAu1yYoH5hc7xDawcYES00VlRtC2Og7hz7ZlQvbE189INTp15g0JuqPF4Rw7mpBmo0fEQG37vz1ACQAEzU8a4Ccsc5n8CR9K4YlVNJQD9b6PffSPnGbbjCOGAPuJWTakj8OUw3q9SgC3qmKxRpEAv-VHY8QeWEQJQgmGFMuifc7waLV~kMaR80J48SnEfulppUAHgjgsXrQ3p2hT3DnKabXmNix~Igj~4rKnDqQcdP9j97ZJp3wne0nu~ElNyb42BHoA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)