After antigen contact, B cells rapidly differentiate and cycle through several phenotypical intermediaries before entering 1 of 2 longer-lived stages, the memory B cell or the plasma cell. In this issue of Blood, van Keimpema et al identify a role for the forkhead transcription factor FOXP1 in inhibiting the very last differentiation stage: plasma cell differentiation.1 They show that FOXP1 directly represses several key regulators of plasma cell differentiation. Although FOXP1 is strongly expressed in naïve B cells, it is lost as B cells enter germinal center differentiation.2 However, it is re-expressed in memory B cells where it correlates with immunoglobulin class switch recombination status, with immunoglobulin (Ig)G-switched memory B cells expressing lower levels of FOXP1. Interestingly, this correlates with a trait of IgG-switched memory B cells to being more likely to enter plasma cell differentiation3 (see figure).
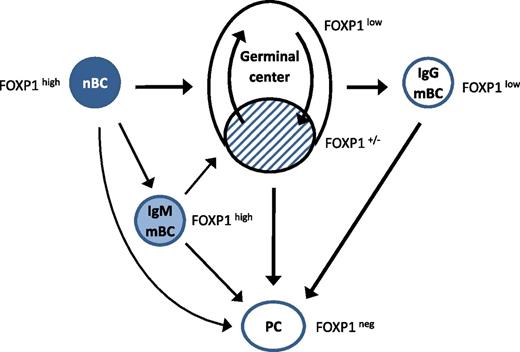
Some of the convoluted pathways of antigen-induced B-cell differentiation. Naïve B cells (nBCs) express high levels of FOXP1. Antigen activation induces formation of germinal centers, extrafollicular plasma cells, and nonswitched memory cells (IgM mBCs) that express intermediate levels of FOXP1. Entry into germinal centers involves loss of FOXP1 expression,2 although some dark zone B cells do express FOXP1 (hatched). IgG-switched memory B cells (IgG mBCs) express lower levels of FOXP1 than nonswitched memory B cells, making them more likely to enter plasma cell differentiation on B-cell receptor stimulation.
Some of the convoluted pathways of antigen-induced B-cell differentiation. Naïve B cells (nBCs) express high levels of FOXP1. Antigen activation induces formation of germinal centers, extrafollicular plasma cells, and nonswitched memory cells (IgM mBCs) that express intermediate levels of FOXP1. Entry into germinal centers involves loss of FOXP1 expression,2 although some dark zone B cells do express FOXP1 (hatched). IgG-switched memory B cells (IgG mBCs) express lower levels of FOXP1 than nonswitched memory B cells, making them more likely to enter plasma cell differentiation on B-cell receptor stimulation.
The forkhead box or FOX proteins are a family of transcriptional regulators, typically displaying the forkhead motive, a 100-amino-acid protein motive involved in DNA binding. The original fork head protein was described in Drosophila melanogaster and is involved in regulating terminal differentiation.4 Immunologists are aware of the family mainly through FOXP3, the master regulator for regulatory T cells.5 Less has been published on FOXP1, which has been largely studied in connection with embryonic and brain development. Similar to other transcription factors, FOXP1 can act as a transcriptional inducer or repressor.2 Transcriptional regulation plays an important role in the development of lymphoma. Increased activities of genes that inhibit terminal plasma cell differentiation, eg, Paired Box 5 (Pax5), B cell lymphoma 6 (Bcl6), Spi-B, or loss of function of Positive Regulatory Domain I (Prdm1), the gene coding for BCL10-interacting MAGUK protein 1 (BLIMP1), are often associated with B-cell non-Hodgkin lymphomas. Similarly, translocation of FoxP1 has been shown to be associated with poor prognosis in diffuse large B-cell lymphoma6 and other lymphomas.
Transcriptional regulation leading to plasma cell differentiation has been intensely studied over recent years. A cascade of transcriptional regulators directs this important process of terminal differentiation, leading to long-term production of protective antibodies. These include interferon regulatory factor 4 (IRF4) upstream of the plasma cell master regulator BLIMP1. BLIMP1 represses and is itself repressed by BCL6, the master regulator of the germinal center program. BLIMP1 also inhibits PAX5, which maintains the B-cell phenotype. PAX5, on the other hand, represses XBP1, which is essential for the secretory phenotype of plasma cells.7 van Keimpena et al show that FOXP1 directly represses Irf4, Prdm1, and Xbp1.
Germinal centers are the sites of immunoglobulin gene hypermutation and B-cell affinity maturation, producing high affinity memory B cells and plasma cells. Cellular differentiation in germinal centers needs to be tightly regulated, as B cells are in a highly activated stage, undergoing intense proliferation and at the same time cycle through various differentiation stages or rapidly enter apoptosis if things are going wrong.8 Germinal center B cells and plasma cells can be seen as 2 extremes of B-cell differentiation, with germinal center B cells rapidly proliferating, differentiating, and migrating, whereas plasma cells typically are secretory, long-lived, nondividing effector cells. As mentioned above, the transcriptional regulatory program in germinal center B cells inhibits regulators of plasma cell differentiation and vice versa.7,8 As plasma cells are a major output from the germinal center, there must be a rapid way to switch from germinal center phenotype into plasma cell differentiation. Interestingly, an earlier study in Blood showed that downregulation of FOXP1 is also essential for entering germinal center differentiation.2 In the light of the present study of van Keimpena et al, this at first seems surprising. Perhaps loss of FOXP1 in germinal center B cells primes B cells to be able to rapidly differentiate into high affinity plasma cells. Plasma cells leave germinal centers via the germinal center dark zone.9 Sagardoy et al noted expression of FOXP1 in some of the B cells of the germinal center dark zone.2 It is possible that in this environment, presumably rich in factors inducing plasma cell differentiation, FOXP1 is expressed in some B cells, preventing their differentiation into plasma cells.
Another key finding of the present study of van Keimpena et al is that IgM- vs IgG-switched memory B cells express different levels of FOXP1. It is well known that IgG-switched memory B cells, more likely to be germinal center derived and of high affinity, have a higher propensity to develop into plasma cells on restimulation.3 However, although the longer cytoplasmic tail of IgG can increase B-cell receptor signaling, the effects of memory type B-cell receptors are complex, and it is not clear whether switched antigen receptor alone can account for the differences in the developmental capacity of IgM- and IgG-switched memory B cells.10 The study presented here for the first time shows that there is different transcriptional programming in class switched memory B cells independent of B-cell receptor-induced activation, which helps explaining the profound differences between switched and nonswitched memory B cells and their capacities to enter plasma cell differentiation or to reenter germinal centers and undergo further affinity maturation.
Conflict-of-interest disclosure: The author declares no competing financial interests.