Key Points
Aberrant expression of FOXP1 in human MBCs represses their ability to differentiate into PCs.
Human IgG+ MBCs combine lower FOXP1 expression with a higher propensity to differentiate as compared with IgM+ MBCs.
Abstract
Expression of the forkhead transcription factor FOXP1 is essential for early B-cell development, whereas downregulation of FOXP1 at the germinal center (GC) stage is required for GC B-cell function. Aberrantly high FOXP1 expression is frequently observed in diffuse large B-cell lymphoma and mucosa-associated lymphoid tissue lymphoma, being associated with poor prognosis. Here, by gene expression analysis upon ectopic overexpression of FOXP1 in primary human memory B cells (MBCs) and B-cell lines, combined with chromatin immunoprecipitation and sequencing, we established that FOXP1 directly represses expression of PRDM1, IRF4, and XBP1, transcriptional master regulators of plasma cell (PC) differentiation. In accordance, FOXP1 is prominently expressed in primary human naive and MBCs, but expression strongly decreases during PC differentiation. Moreover, as compared with immunoglobulin (Ig) M+ MBCs, IgG+ MBCs combine lower expression of FOXP1 with an enhanced intrinsic PC differentiation propensity, and constitutive (over)expression of FOXP1 in B-cell lines and primary human MBCs represses their ability to differentiate into PCs. Taken together, our data indicate that proper control of FOXP1 expression plays a critical role in PC differentiation, whereas aberrant expression of FOXP1 might contribute to lymphomagenesis by blocking this terminal B-cell differentiation.
Introduction
The hallmark of antibody-mediated adaptive immunity is the generation of plasma cells (PCs) producing high titers of antigen-specific antibodies. Upon the first encounter with antigen and T-cell help, naive B cells give rise to a primary immune response. This response results in the generation of short-lived antibody-secreting plasmablasts and germinal center (GC) B cells. In the GCs, the B cells acquire somatic mutations in the variable region of the immunoglobulin genes and can undergo class switch recombination (CSR), whereby the immunoglobulin constant region is switched from immunoglobulin (Ig) M to IgG, IgA, or IgE.1 Cells that acquire somatic mutations that improve antigen-binding affinity gain a survival advantage and emerge from GCs as long-lived memory B cells (MBCs) or PCs, which maintain serum immunoglobulin levels.2 After reexposure to their cognate antigen, MBCs can rapidly differentiate to PCs, resulting in a fast secondary response.
Differentiation of B cells into PCs is regulated by a complex network of transcription factors. IRF4, BLIMP1 (encoded by the PRDM1 gene), and XBP1 are essential drivers of PC differentiation and immunoglobulin secretion,3,4 IRF4 being able to drive expression of BLIMP1,5-8 which in turn induces expression of XBP1.9 Induction of PC differentiation requires an active suppression of the B-cell gene expression program, including BCL6, PAX5, SpiB, and BACH2. These transcription factors inhibit differentiation of activated B cells, allowing sufficient time for affinity maturation and CSR to occur. They act predominantly by repressing the factors required for PC differentiation.4 As such, PC differentiation involves the tight control of expression and coordinated interplay between these transcriptional activators and repressors, including several double-negative feedback mechanisms, for instance PAX5 and BCL6 repressing BLIMP1 expression, and vice versa.10-13 Aberrations in genes that regulate PC differentiation, such as translocations of PAX5 and BCL6, amplification of SPIB, and loss-of-function deletions or mutations in PRDM1, are frequently present in B-cell non-Hodgkin lymphomas.14-17 These aberrations stall further differentiation of the B cells, thereby contributing to lymphomagenesis.18,19
The forkhead transcription factor FOXP1 has been shown to be essential for early B-cell development.20 Recurrent chromosomal translocation involving FOXP1 in diffuse large B-cell lymphoma (DLBCL) and mucosa-associated lymphoid tissue lymphoma, and the frequent aberrantly high FOXP1 expression in these lymphomas, which is associated with poor prognosis, suggest that FOXP1 also exerts functional roles in mature B cells.21-24 In accordance, we recently demonstrated that FOXP1 overexpression in primary human B cells cooperates with nuclear factor κB pathway activity to promote B-cell survival.14,25 Furthermore, a recent study by Sagardoy et al26 showed that FOXP1 expression is temporarily repressed at the GC stage, which is needed for appropriate GC B-cell function.26 However, potential functions of FOXP1 in differentiation of post-GC B cells have not yet been assessed. Here, we show that FOXP1 directly represses expression of essential drivers of PC differentiation, such as PRDM1, IRF4, and XBP1; that FOXP1 is prominently expressed in human naive, GC, and MBCs but not in PCs; and that reduced expression of FOXP1 is required for efficient PC differentiation.
Materials and methods
Constructs
B-cell isolation
Buffy coats were obtained from Sanquin blood bank (Amsterdam, the Netherlands). MBCs were isolated as previously described.25 IgM+ MBCs were isolated by FACSAria sorting of the CD19+CD27+IgG−IgA− population, using an allophycocyanin (APC)-conjugated antibody against CD19 (BD), an fluorescein isothiocyanate (FITC)–conjugated antibody against CD27 (BD), and phycoerythrin (PE)–conjugated antibodies against IgG and IgA (Southern Biotech), and IgG+ MBCs were isolated by FACSAria sorting of the CD19+CD27+IgM−IgA− population, using antibodies against CD19, CD27, and PE-conjugated antibodies against IgM and IgA (Southern Biotech). Tonsils were obtained from children undergoing routine tonsillectomy as previously described.25 Tonsillar B-cell subsets were isolated by FACSAria sorting using an PE-conjugated antibody against CD19 (DAKO), an FITC-conjugated antibody against IgD (Southern Biotech), and an APC-conjugated antibody against CD38 (BD).
Cell culture and retroviral transductions
Microarray analysis, ChIP-seq, and qRT-PCR
Luciferase assay
The BLIMP1-pGL3 construct (Addgene) was used for the luciferase-reporter assay. For details, see supplemental Methods.
Immunoblotting
Samples were applied on a 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis gel and blotted with rabbit anti FOXP1 (Abcam or Cell Signaling), mouse-anti-BCL6 (BD), mouse-anti β-actin or mouse-anti-β-tubulin antibodies (Sigma), followed by horseradish peroxidase–conjugated goat anti-rabbit or goat anti-mouse and developed by enhanced chemiluminescence (Amersham Pharmacia).
ELISPOT
IgG and IgM enzyme-linked immunospot (ELISPOT) assays were performed using IgG and IgM ELISpot kits (Mabtech) according to the manufacturer’s instructions.
ELISA
Enzyme-linked immunosorbent assay (ELISA) was performed essentially as described.34 Details are described in the supplemental Methods.
IgG isotype ELISA was performed using the human IgG subclass profile ELISA kit (Invitrogen) according to the manufacturer’s instructions.
Flow cytometry
Cells were stained with anti-human IgM or IgG (both from Southern Biotech), CD38 (BD), or CD20 conjugated with PE or APC and analyzed on a FACSCanto. For intracellular staining the Foxp3/transcription factor staining buffer set (ebioscience) and anti FOXP1-APC (R&D), CD19-APC-H7, CD27-FITC, and IgM-V450 (all from BD), and IgG-PE were employed.
Results
FOXP1 represses expression of PC signature genes and is prominently expressed in all human mature B-cell subsets except for PCs
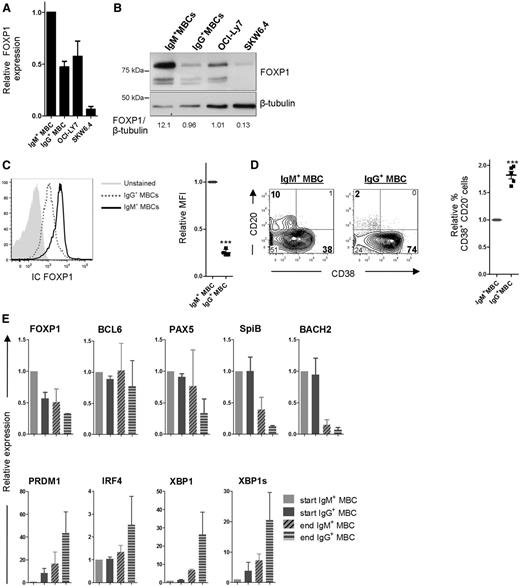
Gene expression microarray analysis of primary human MBCs, retrovirally transduced with LZRS-FOXP1-IRES-YFP to constitutively overexpress FOXP1 or with “empty” expression vector (LZRS-IRES-YFP) as a negative control,25 revealed that FOXP1-downregulated genes were enriched for a previously defined signature of genes highly expressed in PCs (PC-2,35,36 P = .0035; Figure 1A). Among these genes were PRDM1, IRF4, and XBP1, important transcriptional drivers of PC differentiation.
FOXP1 represses the PC gene signature and is expressed in human mature B-cell subsets but not in PCs. (A) Expression of genes that belong to the PC-2 PC gene signature35,36 and were significantly repressed by FOXP1 in microarray analysis of 2 independent experiments of primary human MBCs, transduced with FOXP1 or control vector. Data are represented as z scores. The lower panel shows the mean relative expression values of the gene set. (B-C) Human CD19+ tonsil B-cell subsets, that is, naive (NBC) (IgD+CD38−), transitional (TBC) (IgD+CD38+), GC B (IgD−CD38+), class-switched MBCs (IgD−CD38−), and PCs (IgD−CD38++), and peripheral blood B-cell subsets (MBC [CD27+] and naive enriched [CD27−]) were sorted. (B) Gene and protein expression levels of FOXP1 were analyzed in tonsillar and peripheral blood B-cell subsets. Gene expression levels in tonsillar B-cell subsets were quantified by qRT-PCR and normalized to expression levels in naive B cells. Means ± standard error of the mean (SEM) of 4 independent experiments are shown. Significant differences compared with naive B cells are indicated (1 sample t test, **P < .01). FOXP1 protein expression levels were analyzed by immunoblotting; β-actin was used as loading control. Representative blots of 2 independent experiments are shown. (C) Gene expression levels of BCL6, SPIB, PAX5, PRDM1, IRF4, and XBP1, were analyzed in tonsillar samples by qRT-PCR. Expression levels were normalized to expression levels in naive B cells. Means ± SEM of 4 independent experiments are shown. Significant differences compared with naive B cells are indicated (1 sample t test, *P < .05; **P < .01; ***P < .001).
FOXP1 represses the PC gene signature and is expressed in human mature B-cell subsets but not in PCs. (A) Expression of genes that belong to the PC-2 PC gene signature35,36 and were significantly repressed by FOXP1 in microarray analysis of 2 independent experiments of primary human MBCs, transduced with FOXP1 or control vector. Data are represented as z scores. The lower panel shows the mean relative expression values of the gene set. (B-C) Human CD19+ tonsil B-cell subsets, that is, naive (NBC) (IgD+CD38−), transitional (TBC) (IgD+CD38+), GC B (IgD−CD38+), class-switched MBCs (IgD−CD38−), and PCs (IgD−CD38++), and peripheral blood B-cell subsets (MBC [CD27+] and naive enriched [CD27−]) were sorted. (B) Gene and protein expression levels of FOXP1 were analyzed in tonsillar and peripheral blood B-cell subsets. Gene expression levels in tonsillar B-cell subsets were quantified by qRT-PCR and normalized to expression levels in naive B cells. Means ± standard error of the mean (SEM) of 4 independent experiments are shown. Significant differences compared with naive B cells are indicated (1 sample t test, **P < .01). FOXP1 protein expression levels were analyzed by immunoblotting; β-actin was used as loading control. Representative blots of 2 independent experiments are shown. (C) Gene expression levels of BCL6, SPIB, PAX5, PRDM1, IRF4, and XBP1, were analyzed in tonsillar samples by qRT-PCR. Expression levels were normalized to expression levels in naive B cells. Means ± SEM of 4 independent experiments are shown. Significant differences compared with naive B cells are indicated (1 sample t test, *P < .05; **P < .01; ***P < .001).
FOXP1 has been shown to be expressed in human tonsillar B-cell subsets, but its expression and role in terminally differentiated B cells (ie, PCs) has not been investigated.26 To compare expression levels of FOXP1 in human tonsillar B-cell subsets and PCs, we separated human CD19+ tonsil B cells into naive B cells (IgD+CD38−), transitional B cells (IgD+CD38+), GC B cells (IgD−CD38+), class-switched MBCs (IgD−CD38−), and PCs (IgD−CD38++) by cell sorting. Expression levels of FOXP1 were determined by quantitative real-time PCR and western blotting (Figure 1B). Naive B cells, GC B cells, and MBCs showed prominent expression of FOXP1 messenger RNA (mRNA) and protein. Notably, FOXP1 protein was also prominently expressed in human peripheral blood CD19+CD27− (mainly naive B cells) and CD19+CD27+ (MBCs) B-cell subsets. In contrast, levels of FOXP1 transcripts and protein were very low in PCs. The same samples were also analyzed for mRNA levels of genes that are known to be regulated during PC differentiation. As expected, expression levels of BCL6, PAX5, and SpiB were high in GC B cells but low in PCs, whereas the levels of BLIMP1, IRF4, and XBP1 were highest in PCs (Figure 1C). The observed low expression of FOXP1 in PCs, combined with the repression of the PC gene signature by FOXP1, suggests that FOXP1 downregulation might be required for PC differentiation, whereas aberrantly high expression of FOXP1 might prevent PC differentiation.
FOXP1 arrests differentiation of B-cell lines into antibody-secreting cells and directly represses expression of important transcriptional drivers of differentiation
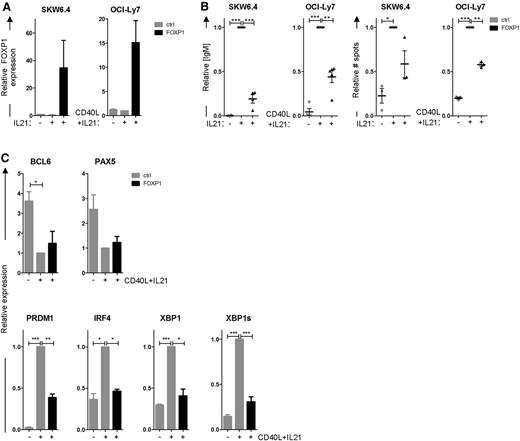
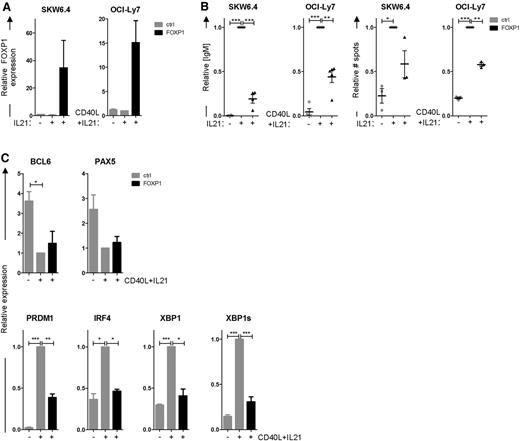
To investigate the putative repressive role of FOXP1 in PC differentiation, we first assessed the effects of ectopic FOXP1 overexpression on in vitro differentiation of B-cell lines. The Epstein-Barr virus (EBV)-transformed B-cell line SKW6.4 and the GC-DLBCL cell line OCI-Ly7 can be induced to differentiate into antibody-secreting cells through stimulation with cytokines.29,30 When cultured in normal culture medium, these cell lines do not secrete immunoglobulins and express low levels of PRDM1, IRF4, and XBP1. Stimulation of these cell lines with interleukin (IL) 21 and CD40 ligand (CD40L)–expressing L cells (OCI-Ly7) or IL-21 alone (SKW6.4) rapidly induced immunoglobulin secretion and the expression of PC-specific genes. Interestingly, ectopic overexpression of FOXP1 in these cell lines (Figure 2A), cultured under these differentiation-inducing conditions, strongly repressed immunoglobulin secretion, as determined by ELISA and ELISPOT (Figure 2B). Moreover, qRT-PCR analysis showed repression of PRDM1, IRF4, and XBP1 induction, as well as the active splice variant of XBP1, in FOXP1-transduced OCI-Ly7 cells, whereas downregulation of PAX5 and BCL6 was not affected (Figure 2C).
FOXP1 represses PC differentiation of SKW6.4 and OCI-LY7 cells. (A-C) SKW6.4 and OCI-Ly7 cells were cultured without stimulation or were stimulated to differentiate with IL-21 and CD40L-L cells (OCI-Ly7) or IL-21 alone (SKW6.4) and were transduced with FOXP1-IRES-YFP or control empty vector. Four (OCI-Ly7) or 6 (SKW6.4) days after transduction, YFP+ cells were sorted. (A) Gene expression levels of FOXP1 as determined by qRT-PCR analysis in sorted cells. Expression levels were normalized to β-actin and then to expression levels in stimulated, control-transduced cells. Means ± SEM of 3 independent experiments are shown. (B) Equal numbers of sorted cells were cultured and analyzed by ELISA (left) or ELISPOT (right). Equal numbers of sorted cells (50 000) were cultured for an additional 24 hours. Thereafter, the supernatants were collected, and IgM protein levels were analyzed by ELISA. Levels were normalized to levels in stimulated, control-transduced cells. Means ± standard deviation (SD) of 5 (OCI-Ly7) or 4 independent experiments are shown. (left). Equal numbers of sorted cells were plated onto membranes in serial dilutions and cultured for an additional 18 hours, after which numbers of IgM-secreting cells were determined by ELISPOT. Spot numbers were normalized to numbers in stimulated, control-transduced cells. Means ± SD of 3 independent experiments are shown (right). (C) Gene expression levels of BCL6, PAX5, PRDM1, IRF4, and XBP1 in OCI-LY7 were analyzed by qRT-PCR. Expression levels were normalized to β-actin and then to expression levels in stimulated, control-transduced cells. Means ± SEM of 3 independent experiments are shown (1 sample t test, *P < .05; **P < .01; ***P < .001).
FOXP1 represses PC differentiation of SKW6.4 and OCI-LY7 cells. (A-C) SKW6.4 and OCI-Ly7 cells were cultured without stimulation or were stimulated to differentiate with IL-21 and CD40L-L cells (OCI-Ly7) or IL-21 alone (SKW6.4) and were transduced with FOXP1-IRES-YFP or control empty vector. Four (OCI-Ly7) or 6 (SKW6.4) days after transduction, YFP+ cells were sorted. (A) Gene expression levels of FOXP1 as determined by qRT-PCR analysis in sorted cells. Expression levels were normalized to β-actin and then to expression levels in stimulated, control-transduced cells. Means ± SEM of 3 independent experiments are shown. (B) Equal numbers of sorted cells were cultured and analyzed by ELISA (left) or ELISPOT (right). Equal numbers of sorted cells (50 000) were cultured for an additional 24 hours. Thereafter, the supernatants were collected, and IgM protein levels were analyzed by ELISA. Levels were normalized to levels in stimulated, control-transduced cells. Means ± standard deviation (SD) of 5 (OCI-Ly7) or 4 independent experiments are shown. (left). Equal numbers of sorted cells were plated onto membranes in serial dilutions and cultured for an additional 18 hours, after which numbers of IgM-secreting cells were determined by ELISPOT. Spot numbers were normalized to numbers in stimulated, control-transduced cells. Means ± SD of 3 independent experiments are shown (right). (C) Gene expression levels of BCL6, PAX5, PRDM1, IRF4, and XBP1 in OCI-LY7 were analyzed by qRT-PCR. Expression levels were normalized to β-actin and then to expression levels in stimulated, control-transduced cells. Means ± SEM of 3 independent experiments are shown (1 sample t test, *P < .05; **P < .01; ***P < .001).
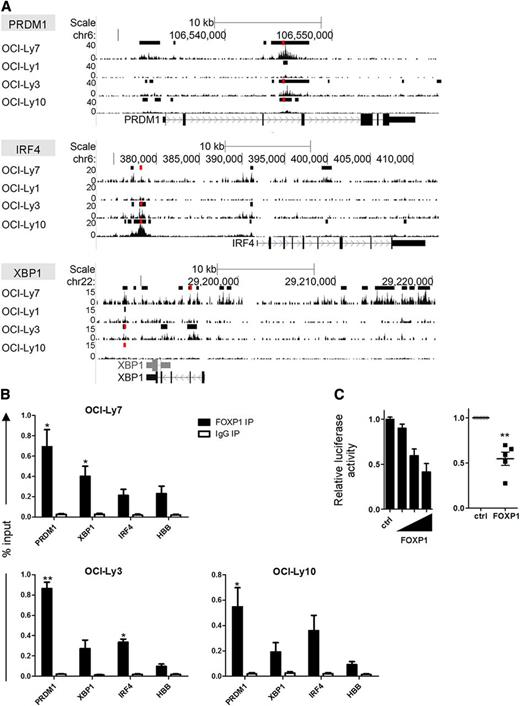
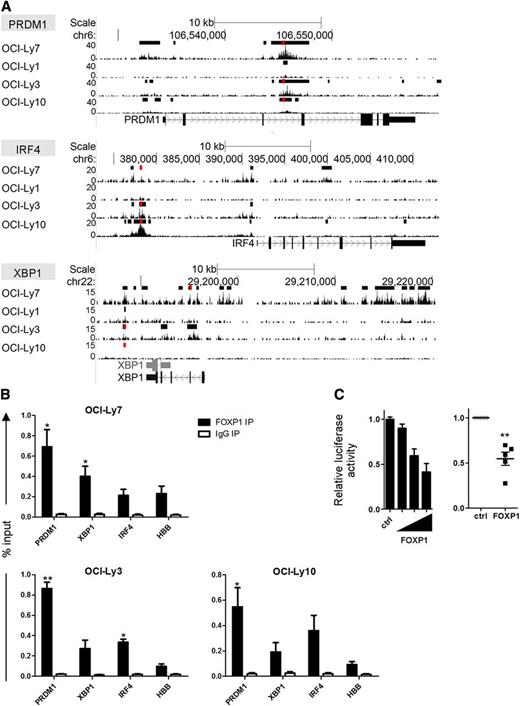
ChIP-seq analysis in OCI-LY7, as well as other DLBCL cell lines, revealed that FOXP1 binds in the vicinity of the transcription start sites (TSSs) of the PRDM1, IRF4, and XBP1 genes (Figure 3). De novo motif search verified the presence of forkhead binding motifs in the FOXP1 binding regions in each of these genes (data not shown).25 Direct binding and repression of the BLIMP1 promoter by FOXP1 was confirmed by a luciferase-reporter assay (Figure 3C). Together, these data indicate that FOXP1 inhibits the capacity of OCI-LY7 and SKW6.4 cells to differentiate into antibody-secreting cells with typical PC features, by directly repressing the upregulation of PRDM1, IRF4, and XBP1.
FOXP1 binds in the proximity of the TSS of PRDM1, IRF4, and XBP1. (A) Tracks showing locations of FOXP1 ChIP-seq peaks in the proximity of the TSS of the PRDM1, IRF4, and XBP1 genes in OCI-Ly7 and 3 other DLBCL cell lines. Called peaks are indicated by black bars above the tracks. (B) ChIP with an antibody against FOXP1 in the DLBCL cell lines OCI-LY7, OCI-LY3, and OCI-LY10, followed by quantitative PCR for regions in the proximity of the PRDM1, XBP1, and IRF4 genes in which FOXP1 ChIP-seq peaks were observed in multiple cell lines (regions are indicated in panel A by red bars above the tracks). Hemoglobin beta (HBB) was taken along as a negative control region. Means ± SEM of at least 3 independent experiments are shown. For all sites analyzed, significant differences were observed between FOXP1 and IgG immunoprecipitation (IP). Significant differences for FOXP1 binding between a specific region and the control region (HBB) are indicated (t test, *P < .05; **P < .01). Primers are listed in supplemental Table 1. (C) A luciferase-reporter construct driven by the PRDM1 promoter was cotransfected in HEK293T cells with a renilla expression vector and increasing (left) or fixed amounts (right) of a FOXP1 expressing vector (FOXP1), or with the empty control vector (ctrl). Values were corrected for renilla luminescence (transfection efficiency) and normalized to expression in control-transduced cells. Means ± SD of a representative experiment of 2 independent experiments performed in triplicate (left), or the means ± SEM of 5 independent experiments (right), are shown.
FOXP1 binds in the proximity of the TSS of PRDM1, IRF4, and XBP1. (A) Tracks showing locations of FOXP1 ChIP-seq peaks in the proximity of the TSS of the PRDM1, IRF4, and XBP1 genes in OCI-Ly7 and 3 other DLBCL cell lines. Called peaks are indicated by black bars above the tracks. (B) ChIP with an antibody against FOXP1 in the DLBCL cell lines OCI-LY7, OCI-LY3, and OCI-LY10, followed by quantitative PCR for regions in the proximity of the PRDM1, XBP1, and IRF4 genes in which FOXP1 ChIP-seq peaks were observed in multiple cell lines (regions are indicated in panel A by red bars above the tracks). Hemoglobin beta (HBB) was taken along as a negative control region. Means ± SEM of at least 3 independent experiments are shown. For all sites analyzed, significant differences were observed between FOXP1 and IgG immunoprecipitation (IP). Significant differences for FOXP1 binding between a specific region and the control region (HBB) are indicated (t test, *P < .05; **P < .01). Primers are listed in supplemental Table 1. (C) A luciferase-reporter construct driven by the PRDM1 promoter was cotransfected in HEK293T cells with a renilla expression vector and increasing (left) or fixed amounts (right) of a FOXP1 expressing vector (FOXP1), or with the empty control vector (ctrl). Values were corrected for renilla luminescence (transfection efficiency) and normalized to expression in control-transduced cells. Means ± SD of a representative experiment of 2 independent experiments performed in triplicate (left), or the means ± SEM of 5 independent experiments (right), are shown.
Constitutive FOXP1 (over)expression represses PC differentiation of primary human MBCs
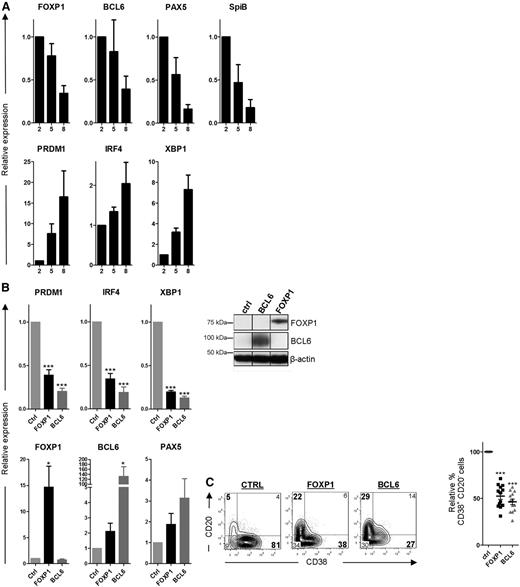
Next, we wanted to assess whether preventing downregulation of FOXP1 might impair differentiation of primary human B cells toward PCs. To this end, CD27+ MBCs were sorted from human peripheral blood and cultured under conditions previously described to drive differentiation toward immunoglobulin-secreting PCs28 : MBCs were first cultured for 5 days on irradiated CD40L-expressing L cells in the presence of IL-21 and IL-2, followed by 3 to 4 days of culture in the presence of IL-21 and IL-2 only. Indeed, the expression of B-cell-specific genes (ie, BCL6, PAX5, and SpiB), including FOXP1, gradually declined, whereas the expression of PC-specific genes (ie, PRDM1, IRF4, and XBP1) increased during consecutive days of the PC differentiation assay (Figure 4A). Furthermore, at the end of the culturing period, a large proportion of the cells had acquired a PC phenotype (CD20−CD38+) and secreted immunoglobulins.
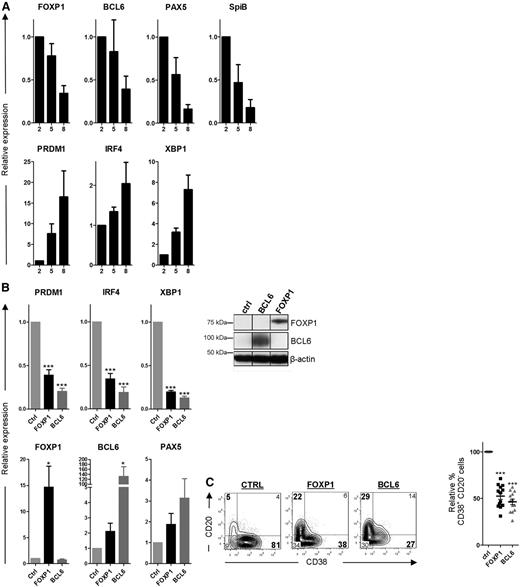
FOXP1 inhibits PC differentiation of primary human MBCs. (A) CD19+CD27+ MBCs were sorted from human peripheral blood and cultured under conditions that promote PC differentiation. Gene expression levels of FOXP1, BCL6, PAX5, SPIB, PRDM1, IRF4, and XBP1 were analyzed by qRT-PCR in samples of cells that were cultured for 2, 5, or 8 days after sorting. Expression levels were normalized to levels in cells that were cultured for 2 days. Means ± SEM of 3 independent experiments are shown. (B) CD19+CD27+ MBCs were sorted from human peripheral blood and transduced with FOXP1-IRES-YFP, BCL6-IRES-GFP, or control empty vector and cultured under conditions that promote PC differentiation. Six days after transduction, yellow fluorescence protein (YFP)- or green fluorescence protein (GFP)-positive cells of cultures transduced with FOXP1, BCL6, or control vector were sorted (left). Gene expression levels of PRDM1, IRF4, XBP1, FOXP1, BCL6, and PAX5 were analyzed by qRT-PCR. Expression levels in FOXP1- and BCL6-transduced cells were normalized to β-actin and then to expression levels in control-transduced cells. Means ± SEM of 5 independent experiments are shown (right). Representative example of FOXP1 and BCL6 overexpression in primary YFP+ or GFP+ B cells as determined by immunoblotting. β-actin was used as loading control. (C) Six days after transduction, YFP- or GFP-positive cells were analyzed for CD20 and CD38 surface expression by flow cytometry. Representative density plots of 1 out of 13 independent experiments are shown (left). Percentages of CD38+CD20− cells in FOXP1- and BCL6-transduced cultures were normalized to control cultures. Means ± SD values of 13 independent experiments are shown (right).
FOXP1 inhibits PC differentiation of primary human MBCs. (A) CD19+CD27+ MBCs were sorted from human peripheral blood and cultured under conditions that promote PC differentiation. Gene expression levels of FOXP1, BCL6, PAX5, SPIB, PRDM1, IRF4, and XBP1 were analyzed by qRT-PCR in samples of cells that were cultured for 2, 5, or 8 days after sorting. Expression levels were normalized to levels in cells that were cultured for 2 days. Means ± SEM of 3 independent experiments are shown. (B) CD19+CD27+ MBCs were sorted from human peripheral blood and transduced with FOXP1-IRES-YFP, BCL6-IRES-GFP, or control empty vector and cultured under conditions that promote PC differentiation. Six days after transduction, yellow fluorescence protein (YFP)- or green fluorescence protein (GFP)-positive cells of cultures transduced with FOXP1, BCL6, or control vector were sorted (left). Gene expression levels of PRDM1, IRF4, XBP1, FOXP1, BCL6, and PAX5 were analyzed by qRT-PCR. Expression levels in FOXP1- and BCL6-transduced cells were normalized to β-actin and then to expression levels in control-transduced cells. Means ± SEM of 5 independent experiments are shown (right). Representative example of FOXP1 and BCL6 overexpression in primary YFP+ or GFP+ B cells as determined by immunoblotting. β-actin was used as loading control. (C) Six days after transduction, YFP- or GFP-positive cells were analyzed for CD20 and CD38 surface expression by flow cytometry. Representative density plots of 1 out of 13 independent experiments are shown (left). Percentages of CD38+CD20− cells in FOXP1- and BCL6-transduced cultures were normalized to control cultures. Means ± SD values of 13 independent experiments are shown (right).
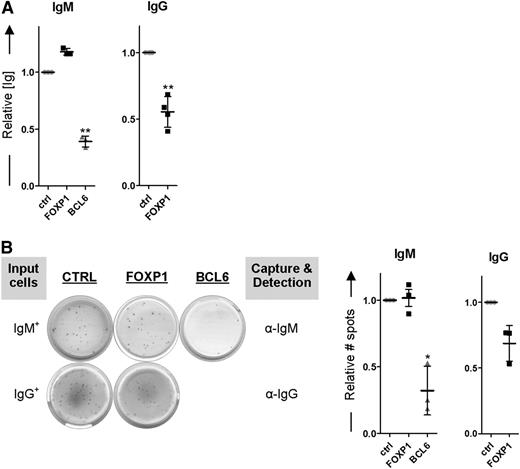
Ectopic overexpression of FOXP1 in these primary human B cells (Figure 4B), cultured under PC differentiation–inducing conditions, resulted in strong repression of PRDM1, IRF4, and XBP1 as determined by qRT-PCR, whereas downregulation of PAX5 and BCL6 was not affected (Figure 4B). A similar repression of the PC signature genes was observed in B cells transduced with BCL6 (Figure 4B), an established inhibitor of in vitro PC differentiation37 (Figure 4C). Moreover, similar to overexpression of BCL6, ectopic overexpression of FOXP1 in these primary B cells resulted in a strong reduction in the formation of CD20−CD38+ PCs (Figure 4C). Importantly, we have previously shown that FOXP1 overexpression does not affect expansion, survival, or proliferation in primary B cells at these culture conditions,25 strongly indicating that the observed effects reflect inhibition of PC differentiation.
In conclusion, FOXP1 directly represses the expression of important transcriptional master regulators of PC differentiation and prevents differentiation of primary human MBCs toward PCs, indicating that FOXP1 downregulation is essential for efficient PC differentiation.
FOXP1 overexpression specifically inhibits formation of IgG- but not IgM-secreting PCs
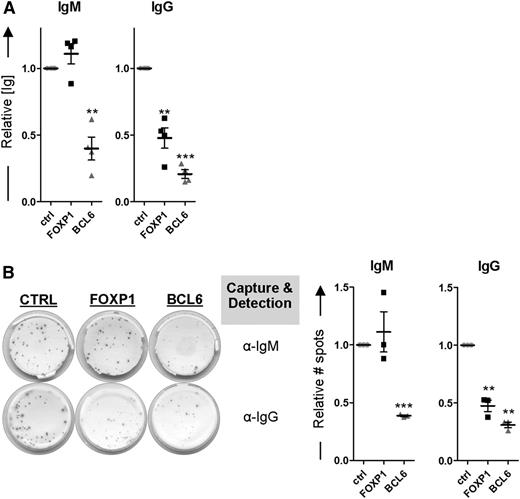
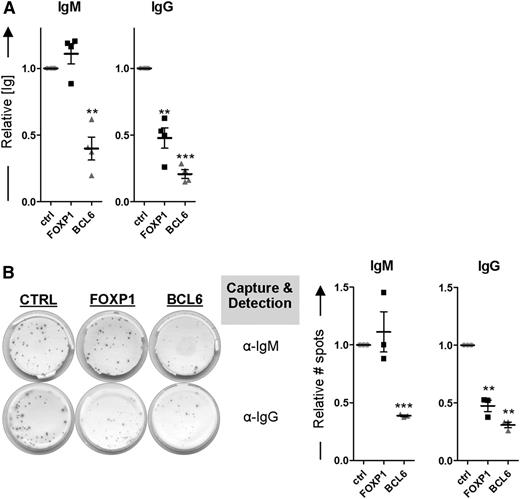
Because FOXP1 inhibits differentiation of primary human MBCs toward PCs, as determined by transcriptional and phenotypic parameters, we anticipated that ectopic expression of FOXP1 in MBCs cultured under PC-promoting conditions would also result in decreased levels of secreted immunoglobulins. Our culture conditions indeed resulted in secretion of IgM and IgG from untransduced cells (Figure 5). Unexpectedly, however, whereas IgG secretion was strongly reduced upon ectopic overexpression of FOXP1, the levels of secreted IgM were not affected (Figure 5A). In contrast, in the culture supernatant of BCL6-transduced MBCs significantly lower levels of both IgG and IgM were detected. Notably, FOXP1 overexpression reduced the secretion of all IgG isotypes (IgG1-4; data not shown). Additional ELISPOT analysis showed that FOXP1 overexpression also exclusively results in a reduction of the number of IgG-secreting cells being formed, whereas BCL6 overexpression caused a reduction in the number of IgM-secreting cells as well (Figure 5B). These results imply that, in contrast to BCL6, overexpression of FOXP1 in primary B cells only impairs the formation of IgG-secreting PCs.
FOXP1 overexpression specifically inhibits formation of IgG- but not IgM-secreting PCs. (A-B) CD19+CD27+ MBCs were sorted from human peripheral blood and transduced with FOXP1-IRES-YFP, BCL6-IRES-GFP, or control empty vector and cultured under conditions that promote PC differentiation. Six days after transduction, YFP- or GFP-positive cells of cultures transduced with FOXP1, BCL6, or control vector were sorted. (A) Equal numbers of sorted cells (50 000) were cultured with IL-21 and IL-2 for an additional 24 hours. Thereafter, the supernatants were collected, and IgM and IgG protein levels were analyzed by ELISA. Levels were normalized to levels in control-transduced cells. Means ± SD of at least 4 independent experiments are shown. (B) Equal numbers of sorted cells were plated onto membranes in serial dilutions and cultured with IL-21 and IL-2 for an additional 18 hours, after which numbers of IgM- or IgG-secreting cells were determined by ELISPOT. Spot numbers were normalized to numbers in stimulated, control-transduced cells. Means ± SD of 3 independent experiments are shown (1 sample t test, *P < .05; **P < .01; ***P < .001).
FOXP1 overexpression specifically inhibits formation of IgG- but not IgM-secreting PCs. (A-B) CD19+CD27+ MBCs were sorted from human peripheral blood and transduced with FOXP1-IRES-YFP, BCL6-IRES-GFP, or control empty vector and cultured under conditions that promote PC differentiation. Six days after transduction, YFP- or GFP-positive cells of cultures transduced with FOXP1, BCL6, or control vector were sorted. (A) Equal numbers of sorted cells (50 000) were cultured with IL-21 and IL-2 for an additional 24 hours. Thereafter, the supernatants were collected, and IgM and IgG protein levels were analyzed by ELISA. Levels were normalized to levels in control-transduced cells. Means ± SD of at least 4 independent experiments are shown. (B) Equal numbers of sorted cells were plated onto membranes in serial dilutions and cultured with IL-21 and IL-2 for an additional 18 hours, after which numbers of IgM- or IgG-secreting cells were determined by ELISPOT. Spot numbers were normalized to numbers in stimulated, control-transduced cells. Means ± SD of 3 independent experiments are shown (1 sample t test, *P < .05; **P < .01; ***P < .001).
The observed exclusive inhibition in formation of IgG-secreting PCs by FOXP1 could very well be because of repression of CSR in our culture system, especially because the majority of the isolated MBCs (± 65%) are unswitsched and express IgM. To assess if MBCs displayed CSR in our culturing system and whether this is affected by ectopic overexpression of FOXP1, we specifically sorted unswitched (IgG−IgA−) MBCs and cultured them under the same PC-inducing conditions as before. Whereas at the start of the assay >99% of the cells were IgM+ MBCs, at the end of the assay 3% to 9% of the cells showed surface IgG or IgA expression (supplemental Figure 1). Thus, although CSR did indeed occur, the percentage of the cells that switched was too small to account for the observed reduced formation of IgG-secreting PCs upon FOXP1 expression. Moreover, the switching efficiency was not affected by ectopic overexpression of FOXP1 (supplemental Figure 1).
IgG+ MBCs combine a reduced expression of FOXP1 with an enhanced propensity to differentiate as compared with IgM+ MBCs
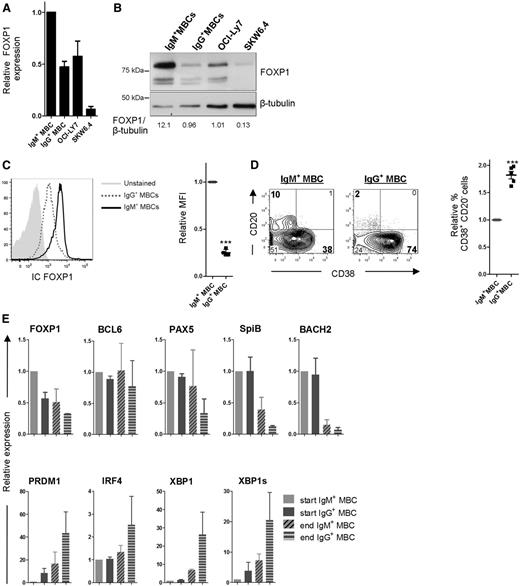
Our results show that ectopic overexpression of FOXP1 specifically represses formation of IgG-secreting PCs in our in vitro culture system, which cannot be explained by impaired class switching. To find a possible explanation for the differential effect of ectopic FOXP1 overexpression on the formation of IgM- vs IgG-secreting PCs, we assessed the expression levels of FOXP1 in IgM+ and IgG+ MBCs. Interestingly, FOXP1 mRNA and protein expression levels were higher in IgM+ MBCs as compared with IgG+ MBCs, as well as the 2 (IgM+) cell lines that displayed impaired PC differentiation upon FOXP1 overexpression (Figure 6A-B). Intracellular flow cytometry analysis demonstrated that the IgM+ MBCs uniformly express higher levels of FOXP1 as compared with IgG+ MBCs (Figure 6C and supplemental Figure 2). Therefore, it is tempting to suggest that the (already) high endogenous expression of FOXP1 in IgM+ MBCs may preclude further repression of their differentiation upon ectopic expression of FOXP1.
IgG+ MBCs display reduced expression of FOXP1 and an enhanced propensity to differentiate as compared with IgM+ MBCs. (A-B) IgM+ (IgG−IgA−) and IgG+ (IgM−IgA−) MBCs were sorted from human peripheral blood and cultured for 2 days after which FOXP1 gene expression levels in these cells, and in OCI-Ly7 and SKW6.4 cells, were compared by qRT-PCR (A) or by immunoblotting (B). (A) Expression levels are normalized to hypoxanthine guanine phosphoribosyl transferase (HPRT) and then to levels in IgM+ MBCs. Means ± SEM of 3 independent experiments are shown. (B) FOXP1 protein expression levels were determined by immunoblotting. β-tubulin levels were determined as loading control. FOXP1 and β-tubulin levels were quantified using Image Studio Lite software, and the ratio of FOXP1 over β-tubulin expression was determined for each lane. A representative blot of 2 independent experiments is shown. (C) PBMCs were isolated from human peripheral blood and stained for surface expression of CD19, CD27, IgM, and IgG, as well as intracellular expression of FOXP1. Intracellular FOXP1 expression in CD19+CD27+IgM+ MBCs and CD19+CD27+IgG+ MBCs was analyzed by flow cytometry. A histogram representative of 4 independent experiments (left), and the mean relative mean fluorescence intensity (MFI) (geometric mean) values ± SD of the IgM+ and IgG+ MBCs (n = 4; right) are shown. MFI values were normalized to values in IgM+ MBCs (1 sample t test, ***P < .001). A specificity control for the intracellular FOXP1 staining is shown in supplemental Figure 2B. (D-E) The sorted cells were cultured under conditions that promote PC differentiation. (D) Eight days after sorting, cells were analyzed for CD20 and CD38 surface expression by flow cytometry. Representative density plots of 1 out of 3 independent experiments are shown (left). Percentages of CD38+ cells were normalized to IgM+ MBCs. Means ± SD values of 3 independent experiments are shown (right). Levels were normalized to levels in IgM+ MBCs (1 sample t test, ***P < .001). (E) Gene expression levels of FOXP1, BCL6, PAX5 SPIB, BACH2, PRDM1, IRF4, and XBP1 were analyzed by qRT-PCR at the start (2 days after sorting) and end (8 days after sorting) of the PC differentiation assay. Expression levels were normalized to expression levels in IgM+ MBCs that had been cultured for 2 days. Means ± SEM of 2 independent experiments performed in triplicate are shown.
IgG+ MBCs display reduced expression of FOXP1 and an enhanced propensity to differentiate as compared with IgM+ MBCs. (A-B) IgM+ (IgG−IgA−) and IgG+ (IgM−IgA−) MBCs were sorted from human peripheral blood and cultured for 2 days after which FOXP1 gene expression levels in these cells, and in OCI-Ly7 and SKW6.4 cells, were compared by qRT-PCR (A) or by immunoblotting (B). (A) Expression levels are normalized to hypoxanthine guanine phosphoribosyl transferase (HPRT) and then to levels in IgM+ MBCs. Means ± SEM of 3 independent experiments are shown. (B) FOXP1 protein expression levels were determined by immunoblotting. β-tubulin levels were determined as loading control. FOXP1 and β-tubulin levels were quantified using Image Studio Lite software, and the ratio of FOXP1 over β-tubulin expression was determined for each lane. A representative blot of 2 independent experiments is shown. (C) PBMCs were isolated from human peripheral blood and stained for surface expression of CD19, CD27, IgM, and IgG, as well as intracellular expression of FOXP1. Intracellular FOXP1 expression in CD19+CD27+IgM+ MBCs and CD19+CD27+IgG+ MBCs was analyzed by flow cytometry. A histogram representative of 4 independent experiments (left), and the mean relative mean fluorescence intensity (MFI) (geometric mean) values ± SD of the IgM+ and IgG+ MBCs (n = 4; right) are shown. MFI values were normalized to values in IgM+ MBCs (1 sample t test, ***P < .001). A specificity control for the intracellular FOXP1 staining is shown in supplemental Figure 2B. (D-E) The sorted cells were cultured under conditions that promote PC differentiation. (D) Eight days after sorting, cells were analyzed for CD20 and CD38 surface expression by flow cytometry. Representative density plots of 1 out of 3 independent experiments are shown (left). Percentages of CD38+ cells were normalized to IgM+ MBCs. Means ± SD values of 3 independent experiments are shown (right). Levels were normalized to levels in IgM+ MBCs (1 sample t test, ***P < .001). (E) Gene expression levels of FOXP1, BCL6, PAX5 SPIB, BACH2, PRDM1, IRF4, and XBP1 were analyzed by qRT-PCR at the start (2 days after sorting) and end (8 days after sorting) of the PC differentiation assay. Expression levels were normalized to expression levels in IgM+ MBCs that had been cultured for 2 days. Means ± SEM of 2 independent experiments performed in triplicate are shown.
If the level of FOXP1 expression in MBCs would indeed control their ability to differentiate into PCs, there should be an intrinsic difference in differentiation propensity of IgM+ (FOXP1high) vs IgG+ (FOXP1low) MBCs. To investigate this, IgG+ (IgM−IgA−) or IgM+ (IgG−IgA−) MBCs were sorted and cultured under PC differentiation-inducing conditions. Strikingly, the percentage of CD20−CD38+ PCs formed was indeed much higher in cultures started from IgG+ MBCs than from IgM+ MBCs (Figure 6C). Furthermore, also the decrease in MBC-specific genes and the concomitant increase in PC-specific genes during culturing was more pronounced in cultures started from IgG+ MBCs (Figure 6D). Of interest, thus far, dissimilar differentiation propensities between IgM+ and IgG+ MBCs have only been reported upon antigen stimulation38,39 ; however, in our culture system the B-cell antigen receptor (BCR) is not activated by antigen. Thus, our results indicate the existence of an antigen/BCR-independent, intrinsic difference in in vitro differentiation propensity of IgM+ vs IgG+ MBCs.
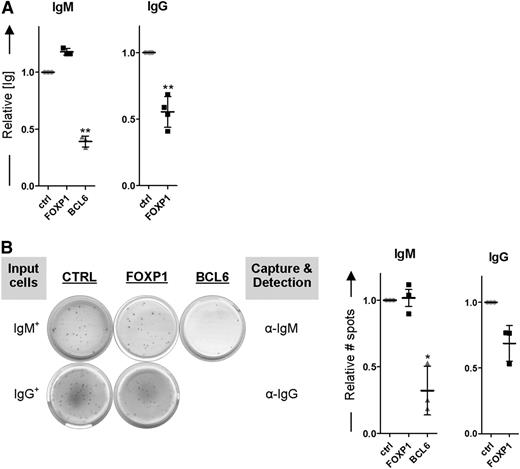
Importantly, whereas FOXP1 expression levels were lower in IgG+ MBCs compared with IgM+ MBCs, no significant differences in expression were observed for other established repressors (BCL6, PAX5, SpiB, Bach2) or drivers (PRDM1, IRF4, XBP1) of PC differentiation (Figure 6D-E). In our analysis, we also included BACH2, which has recently been implicated in dissimilar differentiation potentials of murine naive B cells vs IgG+ MBCs (see “Discussion”)40 ; however, whereas we did observe higher BACH2 expression levels in human naive B cells as compared with human MBCs (supplemental Figure 3), BACH2 expression levels did not differ between human IgM+ and IgG+ MBCs, neither in directly ex vivo purified cells41 (supplemental Figure 3) nor after 2 days of in vitro culture (Figure 6D). To assess if the reduced levels of FOXP1 may be critically required for the enhanced differentiation propensity of IgG+ MBCs, we ectopically overexpressed FOXP1 in sorted IgG+ (IgM−IgA−) MBCs. Indeed, this resulted in reduced (IgG) immunoglobulin secretion and reduced formation of IgG-secreting PCs (Figure 7A-B). In contrast, PC formation in sorted IgM+ MBCs, which already express relatively high levels of FOXP1 and display a reduced PC differentiation propensity, could not be further repressed by ectopic FOXP1 overexpression, whereas BCL6 overexpression repressed differentiation of both MBC subsets (Figure 7A-B). Taken together, our data reveal an enhanced BCR-independent intrinsic PC differentiation propensity of IgG+ vs IgM+ MBCs, which is due to the reduced FOXP1 expression in IgG+ MBCs.
FOXP1 overexpression inhibits differentiation of IgG+ MBCs but cannot further repress differentiation of IgM+ MBCs. IgM+ (IgG−IgA−) and IgG+ (IgM−IgA−) MBCs were sorted from human peripheral blood and transduced with FOXP1-IRES-YFP, BCL6-IRES-GFP (except for IgG+ MBCs), or control empty vector and cultured under conditions that promote PC differentiation. (A) Equal numbers of sorted cells (50 000) were cultured with IL-21 and IL-2 for an additional 24 hours. Thereafter, the supernatants were collected, and IgM and IgG protein levels were analyzed by ELISA. Levels were normalized to levels in control-transduced cells. Means ± SD of 3 independent experiments are shown. (B) Equal numbers of sorted cells were plated onto membranes in serial dilutions and cultured with IL-21 and IL-2 for an additional 18 hours, after which numbers of IgM- or IgG-secreting cells were determined by ELISPOT. Spot numbers were normalized to numbers in stimulated, control-transduced cells. Means ± SD of 3 independent experiments are shown (1 sample t test, *P < .05; **P < .01; ***P < .001).
FOXP1 overexpression inhibits differentiation of IgG+ MBCs but cannot further repress differentiation of IgM+ MBCs. IgM+ (IgG−IgA−) and IgG+ (IgM−IgA−) MBCs were sorted from human peripheral blood and transduced with FOXP1-IRES-YFP, BCL6-IRES-GFP (except for IgG+ MBCs), or control empty vector and cultured under conditions that promote PC differentiation. (A) Equal numbers of sorted cells (50 000) were cultured with IL-21 and IL-2 for an additional 24 hours. Thereafter, the supernatants were collected, and IgM and IgG protein levels were analyzed by ELISA. Levels were normalized to levels in control-transduced cells. Means ± SD of 3 independent experiments are shown. (B) Equal numbers of sorted cells were plated onto membranes in serial dilutions and cultured with IL-21 and IL-2 for an additional 18 hours, after which numbers of IgM- or IgG-secreting cells were determined by ELISPOT. Spot numbers were normalized to numbers in stimulated, control-transduced cells. Means ± SD of 3 independent experiments are shown (1 sample t test, *P < .05; **P < .01; ***P < .001).
Discussion
FOXP1 is prominently expressed in mature B cells and is a potential oncogene in B-cell non-Hodgkin lymphomas; however, the functions of FOXP1 in mature B cells and B-cell lymphomagenesis have not yet been fully explored. Here, by gene expression microarray and ChIP-seq analysis, we demonstrated that FOXP1 directly represses expression of multiple master regulators of PC differentiation. Furthermore, we established that FOXP1 expression strongly declines upon PC differentiation, that constitutive FOXP1 overexpression represses the ability of SKW6.4 and OCI-LY7 cells, as well as primary human MBCs, to differentiate into antibody-secreting PCs; and that IgG+ MBCs combine reduced expression of FOXP1 with an enhanced intrinsic PC differentiation propensity as compared with IgM+ MBCs.
FOXP1 overexpression in primary human MBCs and in the DLBCL cell line OCI-Ly7 resulted in reduced expression of PRDM1, IRF4, and XBP1, important drivers of PC differentiation and immunoglobulin secretion (Figures 2C and 4B). Notably, FOXP1 overexpression did not affect the downregulation of PAX5 and BCL6 during PC differentiation of OCI-LY7 (Figure 2C) and primary human MBCs (Figure 4B). This indicates that the observed FOXP1-mediated repression of IRF4, PRDM1, and XBP1 is the cause rather than a reflection of the impaired PC differentiation, and that FOXP1 can repress PC differentiation despite downregulation of PAX5 and BCL6 (ie, the effect of PAX5 or BCL6 downregulation is overruled by FOXP1 expression). Furthermore, ChIP-seq and ChIP-PCR analysis showed direct binding of FOXP1 in the vicinity of the TSSs of IRF4, PRDM1, and XBP1 in OCI-Ly7 and other DLBCL cell lines (Figure 3A). Moreover, BLIMP1-promoter luciferase-reporter assays confirmed direct repression of the PRDM1gene by FOXP1 by binding to its promoter region. Together, our results indicate that FOXP1 can directly repress transcription of IRF4, PRDM1, and XBP1, irrespective of PAX5 and BCL6, thereby repressing PC differentiation. Because FOXP1 protein expression is temporarily lowered at the GC stage,26 it is tempting to speculate that the extent of FOXP1 upregulation at the end of the GC stage might contribute to the fate of a GC B cell, that is, whether it differentiates into either a PC (no or weak FOXP1 upregulation) or an MBC (strong FOXP1 upregulation).
Strikingly, FOXP1 overexpression inhibited terminal differentiation of primary human IgG+, but, in contrast to BCL6, not of IgM+ MBCs (Figures 5 and 7). This difference does not appear to rely on intrinsic functional differences between the IgM vs the IgG BCR because FOXP1 could also inhibit PC differentiation of IgM-expressing B-cell lines (Figure 2). Rather, the divergent effects on IgG+ and IgM+ MBCs might be the consequence of the dissimilar levels of endogenous FOXP1 protein expression, which is higher in IgM+ MBCs than in IgG+ MBCs and the IgM+ cell lines studied (Figure 6A-B). The high(er) endogenous expression of FOXP1 in IgM+ MBCs appears to be sufficient to fully exert the differentiation inhibiting potential of FOXP1, because, in contrast to ectopic overexpression of BCL6, ectopic overexpression of FOXP1 cannot further reduce the PC differentiation of IgM+ MBCs. Notably, an isotype-specific effect on PC differentiation has previously also been described for the transcription factor STAT3: mice in which this gene is knocked out in B cells showed a specific defect in T-cell–dependent IgG PC formation.42 However, FOXP1 and STAT3 do not seem to act via sequential pathways as STAT3 knockdown in DLBCL cell lines did not affect FOXP1 expression and p-STAT3 expression levels were not affected by FOXP1 overexpression in primary B cells or DLBCL cell lines (data not shown).
In line with our finding that FOXP1 tempers differentiation of MBCs, we found that IgG+ MBCs, which have a twofold lower expression of FOXP1 as compared with IgM+ MBCs, have a clearly increased propensity to differentiate in our in vitro culture system. Previously, differences in the differentiation potential of MBC subsets have been described, but, to the best of our knowledge, exclusively in systems in which, in contrast to our differentiation system, the BCR was triggered by antigenic stimulation.38,39 In these mouse studies, it has been shown that IgG+ MBCs rapidly differentiate into antibody-secreting PCs upon antigenic challenge and do not reenter GCs. In contrast, IgM+ MBCs can return to the GC, whereas only a small proportion of these cells differentiate into PCs.38,39 These unique properties of IgM+ MBCs may be important during reinfection with a mutated version of the original pathogen; by reentering the GC these MBCs could acquire additional mutations in their BCR that would result in a new high-affinity response to the mutated antigen.43 Moreover, because of their long life span, IgM+ MBCs may provide long-lasting immunity, serving as a reservoir of humoral immune memory against a specific antigen, responding when switched immunoglobulin-positive B cells and serum immunoglobulin levels drop below useful levels.39,43
Between human IgM+ and IgG+ MBCs, a similar difference has been proposed to exist: based on replication history and immunoglobulin somatic hypermutation profiles, it has been deduced that CD27+IgM+ MBCs are derived from primary GC responses, whereas at least part of the switched MBCs seem to have undergone multiple rounds of GC passage.44 Furthermore, clonally related IgM+ and IgG+ B cells have been found in human GC and peripheral blood, suggesting that human IgM+ MBCs can indeed reenter GCs, undergo CSR, and exit as switched MBCs.45,46 Traditionally, these differences between IgM+ and IgG+ MBCs have been ascribed to differences in signaling through the unique cytoplasmic tails of IgM and IgG BCR and the higher antigen affinity of IgG vs IgM BCRs39,43,47-50 ; however, our results, obtained in a culture system in which the BCR is not activated by antigen, indicate that antigen-/BCR-independent factors (ie, differential expression of FOXP1) also contribute to these differences. Future studies may reveal the molecular mechanisms underlying this differential expression of FOXP1 among human B-cell and MBC subsets.
In accordance with our findings, in a recent study Zuccarino-Catania et al demonstrated that functionally distinct murine MBC subsets can be defined by expression of surface proteins independent of immunoglobulin isotype, which indicates that the immunoglobulin isotype is just a surrogate marker for MBC subsets and that their terminal differentiation potential might, for example, rather be determined by dissimilar transcription factor expression.51 Likewise, Kometani et al recently reported that although the IgG cytoplasmic tail enhances signaling upon BCR cross-linking,47-50 this alone is insufficient to confer the heightened differentiation activity of murine IgG1 MBCs, compared with naive B cells.40 Rather, repression of the transcription factor Bach2 in antigen-experienced B cells predisposes IgG1 MBCs to differentiate into PCs.40 Our data clearly establish that reduced expression of FOXP1 in human IgG+ MBCs is required for their differentiation into PCs, at least in an in vitro culture system. However, whether the higher FOXP1 expression in IgM+ B cells is also sufficient to repress their differentiation propensity, by directly preventing or delaying upregulation of differentiation master regulators (Figure 6), has to be determined in further studies.
In conclusion, we showed that proper control of FOXP1 expression is critical for MBC fate and PC differentiation. From an oncological perspective, failure to downregulate FOXP1 expression, because of aberrant, constitutively high, expression of FOXP1 by chromosomal translocations or trisomy 3,14,21,22,52,53 will block B-cell differentiation, similar to other established oncogenes and tumor suppressor genes, such as BCL6, SpiB, SOX11, and PRDM1.14,16,54-56 Combined with its recently reported antiapoptotic actions25 (observed in both IgM+ and IgG+ MBCs; data not shown), this would render FOXP1 a highly efficacious oncogene for B-cell lymphomagenesis. From an immunologic perspective, expression of FOXP1 in MBCs might prevent premature differentiation of these cells; specifically, the high expression levels of FOXP1 in IgM+ MBCs could be involved in the reduced propensity of this MBC subset to undergo terminal differentiation, which is suggested to be important for long-lasting immunity,43 whereas reduced expression of FOXP1 in IgG+ MBCs allows their efficient terminal differentiation into antibody-producing PCs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Berend Hooibrink and Toni van Capel for fluorescence-activated cell sorter sorting; Jan Koster and Richard Volckman for providing help with microarray analysis; Edwin Cuppen, Ewart de Bruijn, Nico Lansu, and Wensi Hao for deep sequencing; and Sander Boymans for mapping of the sequencing data.
This work was supported by a grant from the Dutch Cancer Society.
Authorship
Contribution: M.v.K. designed the research, performed experiments, analyzed data, designed figures, and wrote the manuscript; L.J.G., M.M., and R.v.B. performed experiments and analyzed data; M.C.v.Z. and P.C. interpreted data and cosupervised part of the study; S.T.P. cosupervised the study and reviewed the manuscript; and M.S. designed the research, supervised the study, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marcel Spaargaren, Department of Pathology, Academic Medical Center, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; e-mail: marcel.spaargaren@amc.uva.nl.
References
Author notes
S.T.P. and M.S. contributed equally to this study.

![Figure 1. FOXP1 represses the PC gene signature and is expressed in human mature B-cell subsets but not in PCs. (A) Expression of genes that belong to the PC-2 PC gene signature35,36 and were significantly repressed by FOXP1 in microarray analysis of 2 independent experiments of primary human MBCs, transduced with FOXP1 or control vector. Data are represented as z scores. The lower panel shows the mean relative expression values of the gene set. (B-C) Human CD19+ tonsil B-cell subsets, that is, naive (NBC) (IgD+CD38−), transitional (TBC) (IgD+CD38+), GC B (IgD−CD38+), class-switched MBCs (IgD−CD38−), and PCs (IgD−CD38++), and peripheral blood B-cell subsets (MBC [CD27+] and naive enriched [CD27−]) were sorted. (B) Gene and protein expression levels of FOXP1 were analyzed in tonsillar and peripheral blood B-cell subsets. Gene expression levels in tonsillar B-cell subsets were quantified by qRT-PCR and normalized to expression levels in naive B cells. Means ± standard error of the mean (SEM) of 4 independent experiments are shown. Significant differences compared with naive B cells are indicated (1 sample t test, **P < .01). FOXP1 protein expression levels were analyzed by immunoblotting; β-actin was used as loading control. Representative blots of 2 independent experiments are shown. (C) Gene expression levels of BCL6, SPIB, PAX5, PRDM1, IRF4, and XBP1, were analyzed in tonsillar samples by qRT-PCR. Expression levels were normalized to expression levels in naive B cells. Means ± SEM of 4 independent experiments are shown. Significant differences compared with naive B cells are indicated (1 sample t test, *P < .05; **P < .01; ***P < .001).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/126/18/10.1182_blood-2015-02-626176/4/m_2098f1.jpeg?Expires=1768418925&Signature=IKdDemjjeXiifOkkPn~RrEMaovt42liEwf73Pxvp~jUmxQmPERJM8U-2YbKwCM9G1tQuyl3HdgN8YdtPH73xg47tHt9Ic9MfeqLf3tp6m2nvX1tiVdX8Cj~p12raCNsS4juYi0E7xpieGmHJKLQvFekjdPoGaudlLRC9OmoxKCeY3p6unL2TWF5h18QhuTiArHZzad6W8YN~7iomWfXwElreBHxDoCVbNqT2O7jrnjkQEqEB1ZlslBFkvyb1f7Weh3IbUbxq33PQfgtXbMDyY-r2CGm1NSUUw5fqN3QO623lMZBxKXH7FHGU4fHJ1rbjhoXHiPnzVnFeAmMdXCJYPg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 1. FOXP1 represses the PC gene signature and is expressed in human mature B-cell subsets but not in PCs. (A) Expression of genes that belong to the PC-2 PC gene signature35,36 and were significantly repressed by FOXP1 in microarray analysis of 2 independent experiments of primary human MBCs, transduced with FOXP1 or control vector. Data are represented as z scores. The lower panel shows the mean relative expression values of the gene set. (B-C) Human CD19+ tonsil B-cell subsets, that is, naive (NBC) (IgD+CD38−), transitional (TBC) (IgD+CD38+), GC B (IgD−CD38+), class-switched MBCs (IgD−CD38−), and PCs (IgD−CD38++), and peripheral blood B-cell subsets (MBC [CD27+] and naive enriched [CD27−]) were sorted. (B) Gene and protein expression levels of FOXP1 were analyzed in tonsillar and peripheral blood B-cell subsets. Gene expression levels in tonsillar B-cell subsets were quantified by qRT-PCR and normalized to expression levels in naive B cells. Means ± standard error of the mean (SEM) of 4 independent experiments are shown. Significant differences compared with naive B cells are indicated (1 sample t test, **P < .01). FOXP1 protein expression levels were analyzed by immunoblotting; β-actin was used as loading control. Representative blots of 2 independent experiments are shown. (C) Gene expression levels of BCL6, SPIB, PAX5, PRDM1, IRF4, and XBP1, were analyzed in tonsillar samples by qRT-PCR. Expression levels were normalized to expression levels in naive B cells. Means ± SEM of 4 independent experiments are shown. Significant differences compared with naive B cells are indicated (1 sample t test, *P < .05; **P < .01; ***P < .001).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/126/18/10.1182_blood-2015-02-626176/4/m_2098f1.jpeg?Expires=1768786531&Signature=qORxeGlQ8-4bjLxSGeYyxGxQe6MZfOejl1RlSiHKL2yc8FygmgQOKFJNP7FWSeHIhQWNDcALOieyypgZroSvFc22UC9bd0AhEI56o3brDVYAZ0OyUZj1sKXoS5UChk4MWsO9idWLbk4iqbtyFDcN~AER4RCY85LJN2pdMbjCJ6CvPfgqAzljNENDCZBGYyQvFCB9BpORNRathW5CwumRX2k0FnPjPAUqcRE6S8CrQGoYc-XSEjUgJWtf38ofd4TO4eRAn2ccu5bMlenC-FTyoZgDehlQywxqSQlDRgre~~EYRvgIEk1cujMZTjl8YAuKKkagU6Wd9O4UDdbpL526gg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)