In this issue of Blood, Egan et al describe an experiment of nature, whereby gastrointestinal blood loss and related iron deprivation were followed by symptom improvement in a case of congenital erythropoietic porphyria (CEP).1 They then show that prolonged iron deprivation by a chelator, deferasirox, reduces porphyrin overproduction and ineffective erythropoiesis and ameliorates symptoms in both the case and her affected sibling. The authors complement their clinical observation with in vitro investigations showing that erythropoietic precursor cells originating from the CEP cases have an improved survival in an iron-restricted environment.
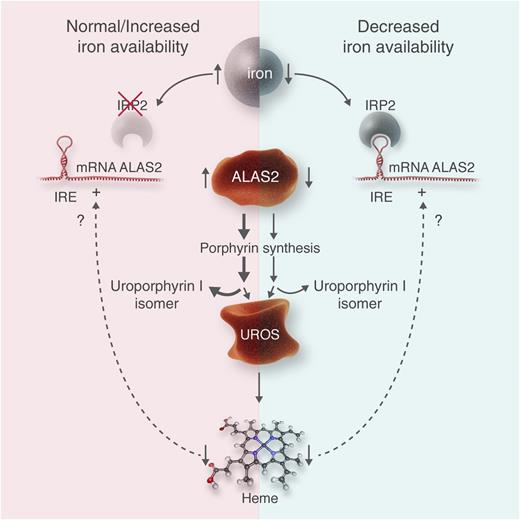
This figure illustrates the hypothesis of Egan et al1 and may be applied analogously to other erythropoietic porphyrias. (Left) A normal to increased iron availability is shown. Thereby, IRP2 is ubiquinated and destroyed, likely by an action of the iron-sensing protein “F-box and leucine-rich repeat protein 5.” IRE in the 5′ end of ALAS2 is unbound, and translation of ALAS2 protein proceeds. This leads to increased activity of this enzyme, controlling the rate of erythropoietic heme synthesis, and an increased overflow of porphyrin intermediaries such as uroporphyrin I in CEP. In CEP, the bottleneck of heme synthesis is the activity of the enzyme uroporphyrinogen III synthase (UROS), whereas in other erythropoietic porphyrias, other enzymes represent the bottlenecks, such as uroporphyrinogen decarboxylase in hepatoerythropoietic porphyria or ferrochelatase in erythropoietic protoporphyria. As shown by Barman-Aksözen et al,6 decreased heme synthesis increases ALAS2 activity by a yet-unknown mechanism. (Right) In an iron-deprived situation, IRP2 is active, binds to the IRE, and blocks ALAS2 translation. As less porphyrin intermediaries are synthesized, their overflow at the bottleneck of UROS is diminished, which reduces symptoms and improves survival of erythrocyte precursor cells in bone marrow. Similar effects of iron deprivation can be expected in other erythropoietic porphyrias. Professional illustration by Luk Cox, Somersault18:24.
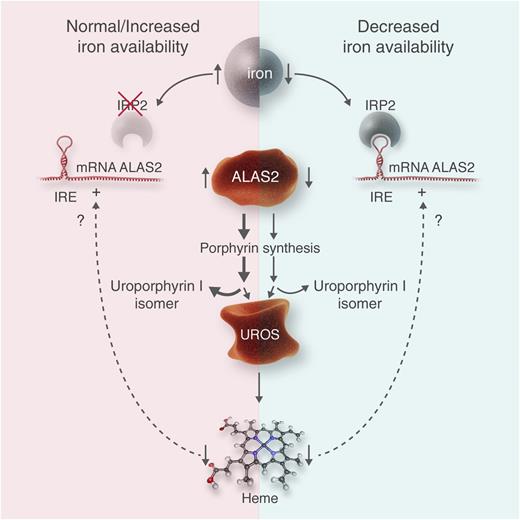
This figure illustrates the hypothesis of Egan et al1 and may be applied analogously to other erythropoietic porphyrias. (Left) A normal to increased iron availability is shown. Thereby, IRP2 is ubiquinated and destroyed, likely by an action of the iron-sensing protein “F-box and leucine-rich repeat protein 5.” IRE in the 5′ end of ALAS2 is unbound, and translation of ALAS2 protein proceeds. This leads to increased activity of this enzyme, controlling the rate of erythropoietic heme synthesis, and an increased overflow of porphyrin intermediaries such as uroporphyrin I in CEP. In CEP, the bottleneck of heme synthesis is the activity of the enzyme uroporphyrinogen III synthase (UROS), whereas in other erythropoietic porphyrias, other enzymes represent the bottlenecks, such as uroporphyrinogen decarboxylase in hepatoerythropoietic porphyria or ferrochelatase in erythropoietic protoporphyria. As shown by Barman-Aksözen et al,6 decreased heme synthesis increases ALAS2 activity by a yet-unknown mechanism. (Right) In an iron-deprived situation, IRP2 is active, binds to the IRE, and blocks ALAS2 translation. As less porphyrin intermediaries are synthesized, their overflow at the bottleneck of UROS is diminished, which reduces symptoms and improves survival of erythrocyte precursor cells in bone marrow. Similar effects of iron deprivation can be expected in other erythropoietic porphyrias. Professional illustration by Luk Cox, Somersault18:24.
Porphyrias are mainly inherited disorders of heme biosynthesis and can be divided into hepatic and erythropoietic forms depending on the tissue of porphyrin overproduction. A major difference between hepatic and erythropoietic heme synthesis is that 2 different genes encode the enzyme of the first and rate-limiting step. In hepatic heme synthesis, it is aminolevulinate-synthase 1, a hepatic and ubiquitous enzyme, and in erythropoiesis heme synthesis, it is aminolevulinate-synthase 2 (ALAS2), which is limited to erythropoiesis. In the liver, the pathway is regulated by the end product heme via a negative feedback mechanism, whereas in erythropoiesis, the regulation is mainly controlled by iron. As shown in the figure, ALAS2 mRNA contains an iron responsive element (IRE) in the 5′ untranslated region. During low iron availability, IRE-binding proteins (mainly homolog 2: IRP2) bind to this IRE, block translation, and diminish ALAS2 activity.2 IRP2 is ubiquitinated if sufficient iron is available. The level of iron likely is sensed by a protein called “F-box and leucine-rich repeat protein 5” that acts as a ubiquitin ligase and leads to the destruction of IRP2.3
ALAS2 became more into focus as an important factor in the erythropoietic porphyrias when Whatley et al4 described X-linked dominant protoporphyria, an erythropoietic porphyria related to gain-of-function mutations in ALAS2, in 2008. Next, To-Figueras et al5 described another gain-of-function mutation in ALAS2 causing a CEP phenotype of increased severity. Recently, our group detected that ALAS2 expression is increased in erythropoietic protoporphyria and that diminished iron availability in those patients is not only associated with a decreased hemoglobin concentration but also with a diminished blood concentration of the relevant protoporphyrin.6 The publication of Egan et al now underlines the importance of ALAS2 activity in the phenotype of CEP, because they show that iron restriction indeed reduced the amount of ALAS2 protein in CEP erythroid bone marrow cells.
Can the conclusions of the authors be generalized that iron restriction may also benefit other CEP patients and patients with other types of erythropoietic porphyrias? CEP is a rare, devastating disorder with a prevalence of 1 per 0.5 to 1.0 million. The severity of CEP varies greatly, whereby the extent of metabolic disturbance, environmental factors (light exposure), and age affect the phenotype. As the rarity of disease has prevented any therapeutic trials, treatment reports have been limited to a single or a few cases. The published options can be separated in those aimed at reducing the metabolic disturbances, preventing the phototoxic skin damage or correcting the sequelae of the disease as outlined by a comprehensive, but retrospective, study of 29 CEP patients.7 Besides strict light avoidance, reflectant sunscreen, β-carotene, or narrow-band UV phototherapy was used to prevent phototoxic damage. Charcoal or cholestyramine was given orally to try to interrupt enterohepatic recirculation of porphyrins, although such an enterohepatic recirculation has never been shown for the water-soluble porphyrin intermediates excessively produced in CEP. The efficacy of all those treatments was ambiguous.7
The different attempts to manipulate erythropoietic heme biosynthesis included hematopoietic stem cell or bone marrow transplantation (BMT) that may be curative. Katugampola et al7 list 16 published cases with a mean observation period of 2.3 years and a mortality rate of 19%. All 13 survivors of this series were apparently well. However, when all 6 cases alive were analyzed in another series,7 serious acute complications were found in all of them. The long-term outcomes included no BMT-related complications in only 3 of 5 patients, progression of CEP in 1 patient, and chronic graft-versus-host reaction in 1 patient. The observation period after BMT in the last patient was only 82 days.
Other means to reduce excess porphyrins include suppression of heme biosynthesis by hypertransfusion8 or hydroxyurea9 to ameliorate the hemolytic anemia by splenectomy or to activate the erythropoiesis by erythropoietin.7 The effect of splenectomy was disappointing.7 Only a single case report with short-term hydroxyurea treatment has been published.9 Hypertransfusion in CEP patients inevitably leads to iron overload, and these patients require chelation therapy.7 Some of them received erythropoietin, which apparently induced some improvement of skin symptoms for a limited period.7 This short summary emphasizes that available treatment options in CEP are far from satisfactory; therefore, new approaches as suggested by Egan et al are indeed welcome. Considering their data, hypertransfusion in CEP needs reevaluation, as it may induce a vicious circle of iron overload, overactivity of ALAS2, augmented porphyrin excess, and ineffective erythropoiesis. In symptomatic anemia, a combination of iron chelation with erythropoietin could be attempted, as 2 of 3 CEP patients, and 1 patient with hepato-erythropoietic porphyria, reported improved wound healing.7,10 The symptoms of 1 of the 3 CEP patients on erythropoietin deteriorated after we added iron supplements, an observation also supporting the hypothesis of Egan et al1 (E.I.M., unpublished data, 2004).
Other erythropoietic porphyrias could profit from iron chelation as well. In the rare complication of liver failure in erythropoietic protoporphyria, where only liver transplantation is life saving, an attempt to reduce ALAS2 overactivity, excess protoporphyrin production, and intrahepatic protoporphyrin accumulation by iron chelation can be considered based on data from Barman-Aksözen et al.6
However, caution is needed, as iron chelators have been evaluated for iron overload only; interruption of their application is recommended when ferritin falls to <500 µg/L (deferiprone, deferasirox) or 1000 µg/L (deferrioxamin), and the long-term adverse effects of iatrogenic iron deficiency induced by chelators remains unknown. Treating CEP and other erythropoietic porphyrias with iron chelation could be as treacherous as sailing between Scylla and Charybdis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.