Key Points
Hematologic effects in the mouse model for ARC syndrome, Vps33bfl/fl-ERT2, in which Vps33b is ubiquitously excised post-development.
The VPS33B-VIPAR complex is responsible for sorting cargo to and maturation of α-granule-destined MVBs.
Abstract
Arthrogryposis, renal dysfunction, and cholestasis (ARC) syndrome is caused by deficiencies in the trafficking proteins VPS33B or VIPAR, and is associated with a bleeding diathesis and a marked reduction in platelet α-granules. We generated a tamoxifen-inducible mouse model of VPS33B deficiency, Vps33bfl/fl-ERT2, and studied the platelet phenotype and α-granule biogenesis. Ultrastructural analysis of Vps33bfl/fl-ERT2 platelets identified a marked reduction in α-granule count and the presence of small granule-like structures in agreement with the platelet phenotype observed in ARC patients. A reduction of ∼65% to 75% was observed in the α-granule proteins von Willebrand factor and P-selectin. Although platelet aggregation responses were not affected, a defect in δ-granule secretion was observed. Under arteriolar shear conditions, Vps33bfl/fl-ERT2 platelets were unable to form stable aggregates, and tail-bleeding measurement revealed a bleeding diathesis. Analysis of bone marrow-derived megakaryocytes (MKs) by conventional and immuno-electron microscopy from Vps33bfl/fl-ERT2 mice revealed a reduction in mature type-II multivesicular bodies (MVB II) and an accumulation of large vacuoles. Proteins that are normally stored in α-granules were underrepresented in MVB II and proplatelet extensions. These results demonstrate that abnormal protein trafficking and impairment in MVB maturation in MKs underlie the α-granule deficiency in Vps33bfl/fl-ERT2 mouse and ARC patients.
Introduction
Platelet α-granules contain over 250 proteins that participate in a diverse range of vital processes including hemostasis, tissue repair, angiogenesis, inflammation, and host defense.1-3 α-Granules form in the megakaryocyte (MK) and their maturation continues in the circulating platelet by constitutive endocytosis.4-6 During MK development, α-granule cargo synthesized in the trans-Golgi network or derived from endocytosis of plasma membranes is trafficked to multivesicular bodies (MVBs).3,7 Kinetic studies in MKs have demonstrated that MVBs are a subset of late endosomes that have undergone internal vesicle budding (MVB I) and further maturation (MVB II), with delivery of newly synthesized proteins leading to α-granule formation.8 Although little is known about the intracellular trafficking of proteins in MKs, experiments using ultrathin cryosectioning and immuno-electron microscopy (IEM) suggest that MVBs are an intermediate stage in the formation of α-granules.8
Several insights into platelet α-granule biogenesis have come from studying patients with Gray Platelet Syndrome (GPS, MIM 139090). GPS is characterized by variable thrombocytopenia and absence of platelet α-granules. Mutations in NBEAL29-11 or GFI1B12,13 have been identified in some of the patients, however, the exact roles of those proteins in α-granules production are not clear. Studies using established mouse models that mimic the GPS phenotype (Nbeal2−/− mice) have revealed that MKs underwent abnormal maturation and platelets lacked α-granules. These mice have defective hemostasis and thrombosis, as well as altered thrombo-inflammatory disease states and tissue repair after injury.14-17 Interestingly, the most recent study17 showed that MKs from NBEAL2-deficient mice contained α-granule numbers comparable to controls, which were lost after proplatelet formation.
Another inherited disorder where an absence of α-granules is observed is Arthrogryposis, Renal dysfunction, and Cholestasis syndrome (ARC, MIM 208085). ARC is a rare autosomal recessive multisystem disorder characterized by developmental and functional defects in several organs. The majority of reported patients with ARC died in infancy due to metabolic decompensation or bleeding related to intercurrent illness.18 ARC is caused by mutations in VPS33B or VIPAS39 that encode the trafficking proteins VPS33B and VIPAR, respectively, which together form a functional complex.19-21 Agranular platelets in patients with ARC phenotype were first described in 1990.22 Subsequent studies in patients with mutations in VPS33B confirmed the absence of platelet α-granules, including deficiencies in endogenously synthesized and endocytosed α-granule proteins in this disorder.18,23-25 A previous study showed that half of the patients with ARC developed life-threatening hemorrhage after invasive procedures such as organ biopsies.18 The involvement of VPS33B and its interacting partner, VIPAR, in α-granule formation is still poorly understood.
In the present study, we have generated a tamoxifen-inducible mouse model of VPS33B deficiency in order to investigate the molecular basis of the defect in α-granule biogenesis. Here, for the first time, we demonstrate abnormal protein content in MVBs, as well as deficiency in α-granule production in VPS33B-deficient mice, which suggests that VPS33B regulates protein sorting into α-granule destined organelles. Our results lead us to conclude that VPS33B is a key regulator of MVB maturation during megakaryopoiesis.
Methods
Vps33bfl/fl-ERT2 mouse generation
Conditional Vps33b embryonic stem cell lines and subsequent mice with LoxP sites flanking Vps33b exons 2-3 were developed by Artemis Pharmaceuticals (Cologne, Germany). Heterozygous Vps33b+/fl mice were crossed in order to produce Vps33bfl/fl mice on a C57BL/6J background. Vps33bfl/fl mice were further crossed with CreERT2-recombinase expressing mice (The Jackson Laboratory, Bar Harbor, ME) to obtain Vps33bfl/fl-ERT2. CreERT2-recombinase expression for removal of Vps33b exons 2-3 was induced by intraperitoneal (IP) injections of tamoxifen 100 mg/kg per day (Sigma-Aldrich, Dorset, United Kingdom) on 5 consecutive days at 6 to 8 weeks of age, and subsequent platelet analysis was carried out 5 weeks post-induction. The rationale behind tamoxifen dosage and timing selection is discussed in supplemental Methods, available on the Blood Web site. Age-matched littermates were used as control mice referred to as Vps33bfl/fl that were either: (1) CreERT2+ untreated; or (2) CreERT2− tamoxifen-treated Vps33bfl/fl mice. Data from controls was pooled due to invariability of results. All procedures were undertaken with the United Kingdom Home Office approval in accordance with the Animals (Scientific Procedures) Act of 1986.
Patients
Four unrelated patients with classical features of ARC were recruited for this study. These included 3 patients with mutations in VPS33B: (Patient 1) compound heterozygous for c.745G>T; p.G249C and c.1235_1236delinsG; p.Pro412Argfs*7; (Patient 2) compound heterozygous for c.1225+5G>C and c.440_449del; p.Pro147Argfs*4; (Patient 3) homozygous for p.Trp534*; and (Patient 4) containing the homozygous p.Arg270* VIPAS39 mutation and underwent platelet testing. Patient research was approved by the South Birmingham (REC CA/5175, 06/MRE07/36) and London (REC 13/LO/0168) Research Ethics Committees. Informed consent was provided according to the Declaration of Helsinki.
Statistical analysis
Results are shown as mean ± SEM unless otherwise stated. Statistical analysis was performed with GraphPad Prism 5 software (San Diego, CA) using the Student t test or Mann–Whitney test. P < .05 was considered as statistically significant.
Quantitative real-time polymerase chain reaction, immunohistochemistry, platelet preparation, platelet aggregation and secretion, platelet half-life assay, platelet adhesion under flow, bleeding time assay, MK culture, electron microscopy (EM), flow cytometry, immunoblot analysis, and immunofluorescence microscopy are described in supplemental Methods.
Results
Characterization of Vps33bfl/fl-ERT2 mice
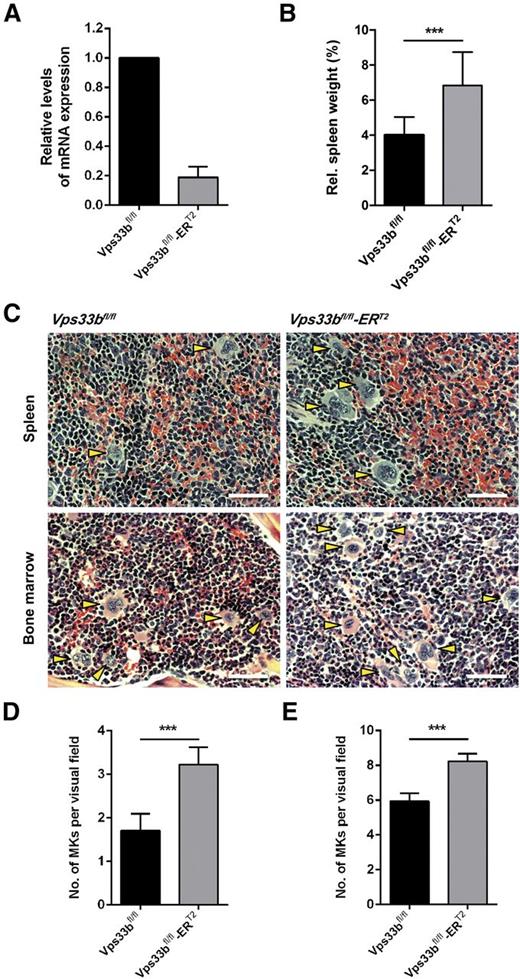
A tamoxifen-inducible Vps33b mouse, Vps33bfl/fl-ERT2, was generated for global deletion of the Vps33b gene in adult mice. CreERT2-recombinase mediated Vps33b excision of exons 2-3 in the Vps33bfl/fl mouse results in frameshift and premature termination of transcription (supplemental Figure 1A). Tamoxifen induction was performed by IP injections over 5 days in 6- to 8-week-old mice with analysis of mice at 5 weeks post-induction (supplemental Figure 1B). At that stage, Vps33bfl/fl-ERT2 mice had begun to develop dry skin and mild-to-severe scaling with occasional appearance of macerated skin lesions (supplemental Figure 1C). Vps33b excision was shown in bone marrow (BM)-derived MKs by quantitative reverse-transcription polymerase chain reaction (Figure 1A). At 5 weeks post-induction, transcription of Vps33b was reduced by 81% ± 7.4 (n = 4) in MKs compared with age-matched littermate controls.
Characterization of the Vps33bfl/fl-ERT2 mice. (A) Analysis of Vps33b messenger RNA in BM-derived MKs of Vps33bfl/fl and Vps33bfl/fl-ERT2 mice. (B) Spleen to body weight ratio was analyzed in 11-week-old mice 5 weeks post-induction (n = 12 to 16 mice per genotype). (C) Extramedullary hematopoiesis in Vps33bfl/fl-ERT2 mice. Representative images of hematoxylin and eosin-stained spleen (top panels) and femur BM (bottom panels) sections from littermate controls (left top and bottom panels) and Vps33bfl/fl-ERT2 mice (right top and bottom panels). MKs are indicated by yellow arrowheads (n = 3 mice per genotype). Bright field images were obtained using a Zeiss Axiovert 200 inverted high-end microscope with a 20× objective. Scale bar, 50 μm. (D-E) Determination of MK numbers per visual field (294 × 221 μm, 36 fields of view from 3 mice per genotype) in hematoxylin and eosin-stained spleen (D) and BM (E) sections of Vps33bfl/fl vs Vps33bfl/fl-ERT2 mice. Mean ± SEM. All values are mean ± standard deviation (SD) unless otherwise indicated. ***P < .001.
Characterization of the Vps33bfl/fl-ERT2 mice. (A) Analysis of Vps33b messenger RNA in BM-derived MKs of Vps33bfl/fl and Vps33bfl/fl-ERT2 mice. (B) Spleen to body weight ratio was analyzed in 11-week-old mice 5 weeks post-induction (n = 12 to 16 mice per genotype). (C) Extramedullary hematopoiesis in Vps33bfl/fl-ERT2 mice. Representative images of hematoxylin and eosin-stained spleen (top panels) and femur BM (bottom panels) sections from littermate controls (left top and bottom panels) and Vps33bfl/fl-ERT2 mice (right top and bottom panels). MKs are indicated by yellow arrowheads (n = 3 mice per genotype). Bright field images were obtained using a Zeiss Axiovert 200 inverted high-end microscope with a 20× objective. Scale bar, 50 μm. (D-E) Determination of MK numbers per visual field (294 × 221 μm, 36 fields of view from 3 mice per genotype) in hematoxylin and eosin-stained spleen (D) and BM (E) sections of Vps33bfl/fl vs Vps33bfl/fl-ERT2 mice. Mean ± SEM. All values are mean ± standard deviation (SD) unless otherwise indicated. ***P < .001.
Vps33bfl/fl-ERT2 mice have an increased platelet count and deficiency in α-granules
Whole blood analysis revealed a 27% increase in platelet count accompanied by a small increase in platelet volume in Vps33bfl/fl-ERT2 mice (Table 1). This elevated platelet count was not due to reduced clearance in Vps33bfl/fl-ERT2 mice as shown by the measurement of the platelet half-life (supplemental Figure 2). Vps33bfl/fl-ERT2 mice also exhibited a 12% reduction in lymphocytes, a 120% increase in monocytes, and a 38% increase in neutrophils when compared with Vps33bfl/fl mice (Table 1). The spleen, which acts as a site of platelet clearance and, if required, production, was enlarged by 70% in Vps33bfl/fl-ERT2 mice compared with littermate controls (n = 12 to 16) (Figure 1B). Immunophenotyping analysis of splenic cells by flow cytometry showed a 51% decrease in dendritic cells and a 231% increase in macrophages (supplemental Table 1). Histologic examination of spleen sections revealed an increase in the number of MKs in the red pulp (Vps33bfl/fl: 1.7 ± 0.4 per visual field; Vps33bfl/fl-ERT2: 3.2 ± 0.4 per visual field; P < .001), indicative of extramedullary hematopoiesis (n = 36 fields of view from 3 mice per genotype) (Figure 1C-D). An increase in MKs was also observed in the BM of Vps33bfl/fl-ERT2 mice (8.2 ± 0.4 per visual field) when compared with controls (5.9 ± 0.4 per visual field) (n = 36 fields of view from 3 mice per genotype; P < .001) (Figure 1C,E). The increase in the number of MKs is likely to account for the raised platelet count.
Basic blood parameters of Vps33bfl/fl-ERT2 mice
| Hematological parameters . | Vps33bfl/fl (Mean ± SEM; n = 35) . | Vps33bfl/fl-ERT2 (Mean ± SEM; n = 26) . |
|---|---|---|
| PLT (×103/μL) | 893.5 ± 160.6 | 1136.0 ± 160.4*** |
| MPV (fL) | 5.5 ± 0.4 | 5.9 ± 0.5** |
| WBC (×103/μL) | 4.1 ± 0.3 | 5.2 ± 0.4 |
| RBC (×106/μL) | 6.2 ± 0.3 | 5.9 ± 0.1 |
| HCT (%) | 29.4 ± 0.6 | 28.1 ± 0.7 |
| LYM (%) | 80.7 ± 1.6 | 70.9 ± 2.3*** |
| MON (%) | 7.3 ± 0.9 | 16.0 ± 2.0*** |
| NEU (%) | 8.0 ± 0.5 | 11.0 ± 0.9** |
| EOS (%) | 0.7 ± 0.2 | 0.9 ± 0.3 |
| BAS (%) | 0.7 ± 0.2 | 1.1 ± 0.4 |
| Hematological parameters . | Vps33bfl/fl (Mean ± SEM; n = 35) . | Vps33bfl/fl-ERT2 (Mean ± SEM; n = 26) . |
|---|---|---|
| PLT (×103/μL) | 893.5 ± 160.6 | 1136.0 ± 160.4*** |
| MPV (fL) | 5.5 ± 0.4 | 5.9 ± 0.5** |
| WBC (×103/μL) | 4.1 ± 0.3 | 5.2 ± 0.4 |
| RBC (×106/μL) | 6.2 ± 0.3 | 5.9 ± 0.1 |
| HCT (%) | 29.4 ± 0.6 | 28.1 ± 0.7 |
| LYM (%) | 80.7 ± 1.6 | 70.9 ± 2.3*** |
| MON (%) | 7.3 ± 0.9 | 16.0 ± 2.0*** |
| NEU (%) | 8.0 ± 0.5 | 11.0 ± 0.9** |
| EOS (%) | 0.7 ± 0.2 | 0.9 ± 0.3 |
| BAS (%) | 0.7 ± 0.2 | 1.1 ± 0.4 |
Diluted whole blood was analyzed using an ABX Pentra 60 hematology analyzer.
BAS, basophils; EOS, eosinophils; HCT, hematocrit; LYM, lymphocytes; MON, monocytes; MPV, mean platelet volume; NEU, neutrophils; PLT, platelets; RBC, red blood cells; WBC, white blood cells.
**P < .01; ***P < .001.
Ultrastructural analysis using transmission EM (TEM) revealed 2 discrete populations of platelets in Vps33bfl/fl-ERT2 mice, with α-granules absent in ∼75% of the Vps33bfl/fl-ERT2 platelets (Figure 2A-B). The remaining Vps33bfl/fl-ERT2 platelets had a similar number and morphology of α-granules to that in controls (supplemental Figure 3A). The presence of the latter population may be due to the existence of MK precursors that have escaped CreERT2-recombinase Vps33b excision. Whole mount EM showed that the number of δ-granules was not altered in Vps33bfl/fl-ERT2 platelets (Vps33bfl/fl: 4.6 ± 0.8 per platelet; Vps33bfl/fl-ERT2: 4.9 ± 0.5 per platelet, n = 30 platelets) (supplemental Figure 3B-C). The appearance of small α-granule–like structures could be seen in some platelets of Vps33bfl/fl-ERT2 mice that lack α-granules (Figure 2C). These structures were also observed in 2 ARC patients with mutations in VPS33B and 1 patient with a mutation in VIPAS39, but were absent in controls (Figure 2D). Over 70% of the patients’ platelets were devoid of α-granules, in agreement with previous reports (supplemental Figure 4).23
Ultrastructural analysis of Vps33bfl/fl-ERT2 platelets reveals an α-granule deficiency. (A) Representative transmission electron micrographs of platelets from Vps33bfl/fl (left panels) and Vps33bfl/fl-ERT2 (right panels) mice at low (scale bar, 1 μm) and high magnification (scale bar, 0.5 μm). Images were obtained using a Tecnai G2 Spirit TEM. (B) Percentage of α-granule containing platelets per ultrathin section (70 to 90 nm) in Vps33bfl/fl and Vps33bfl/fl-ERT2 mice (n = 200 platelets per genotype). (C) Platelets containing small granules were observed in TEM from Vps33bfl/fl-ERT2 mice. Those platelets were devoid of α-granules. Scale bar, 0.5 μm. (D) Representative transmission electron micrographs of an ARC patient with a VIPAS39 mutation (p.Arg270*, top right panel) and 2 ARC patients with VPS33B mutations (p.Pro412Argfs*7 or p.Pro147Argfs*4, bottom left and right panel respectively). Small α-granule–like structures are shown in white arrowheads. Scale bar, 1 μm. (E) Immunogold labeling (IEM) using the Tokuyasu method for the presence of VWF (10 nm gold) in platelets from Vps33bfl/fl (left panel) and Vps33bfl/fl-ERT2 (right panel) mice. Scale bar, 200 nm. All values are mean ± SD. ***P < .001. α, α-granule; δ, δ-granule; G, α-granule-like structure; M, mitochondrion.
Ultrastructural analysis of Vps33bfl/fl-ERT2 platelets reveals an α-granule deficiency. (A) Representative transmission electron micrographs of platelets from Vps33bfl/fl (left panels) and Vps33bfl/fl-ERT2 (right panels) mice at low (scale bar, 1 μm) and high magnification (scale bar, 0.5 μm). Images were obtained using a Tecnai G2 Spirit TEM. (B) Percentage of α-granule containing platelets per ultrathin section (70 to 90 nm) in Vps33bfl/fl and Vps33bfl/fl-ERT2 mice (n = 200 platelets per genotype). (C) Platelets containing small granules were observed in TEM from Vps33bfl/fl-ERT2 mice. Those platelets were devoid of α-granules. Scale bar, 0.5 μm. (D) Representative transmission electron micrographs of an ARC patient with a VIPAS39 mutation (p.Arg270*, top right panel) and 2 ARC patients with VPS33B mutations (p.Pro412Argfs*7 or p.Pro147Argfs*4, bottom left and right panel respectively). Small α-granule–like structures are shown in white arrowheads. Scale bar, 1 μm. (E) Immunogold labeling (IEM) using the Tokuyasu method for the presence of VWF (10 nm gold) in platelets from Vps33bfl/fl (left panel) and Vps33bfl/fl-ERT2 (right panel) mice. Scale bar, 200 nm. All values are mean ± SD. ***P < .001. α, α-granule; δ, δ-granule; G, α-granule-like structure; M, mitochondrion.
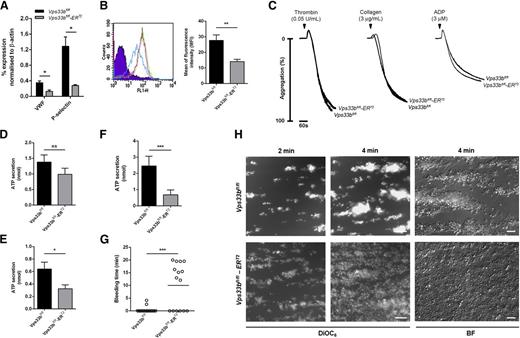
Immunogold labeling on cryosections for von Willebrand factor (VWF), a protein stored in α-granules, showed the presence of this secreted protein in the small α-granule–like structures (Figure 2E) that were observed by TEM, suggesting that these granules could be a consequence of defective α-granule formation. Moreover, platelet VWF content determined by immunoblotting and densitometry (Figure 3A) was reduced by ∼65% in Vps33bfl/fl-ERT2 platelets compared with controls (n = 3 to 5; P < .05). Similarly, a significant reduction (∼75%) was observed in P-selectin content (Figure 3A). Flow cytometric analysis measuring P-selectin surface expression in washed platelets was used as a marker for α-granule release. P-selectin surface expression in response to thrombin (0.1 U/mL) was reduced by ∼50% in Vps33bfl/fl-ERT2 platelets compared with controls (n = 15 to 19; P < .01) (Figure 3B). These results are consistent with abnormal α-granule biogenesis and protein packaging during MK maturation. The subpopulation of Vps33bfl/fl-ERT2 platelets with normal α-granules and the localization of cargo proteins to alternative compartments such as the small granules may explain why the difference in VWF and P-selectin levels is not more pronounced.
Characterization of Vps33bfl/fl-ERT2 platelet function. (A) VWF and P-selectin levels were measured in platelets by immunoblot analysis. β-Actin was used as a loading control. Densitometric analysis showed a reduction in VWF and P-selectin content in Vps33bfl/fl-ERT2 platelets (n = 3 mice) in comparison with Vps33bfl/fl platelets (n = 5 mice). (B) Thrombin (0.1 U/mL) induced P-selectin expression on the surface of Vps33bfl/fl-ERT2 (n = 15 mice) platelets in comparison with Vps33bfl/fl (n = 19 mice). Histogram (left) shows an IgG control (purple), P-selectin expression in controls (green and pink), and Vps33bfl/fl-ERT2 platelets (blue). (C) Representative aggregation responses to thrombin (0.05 U/mL), collagen (3 μg/mL), and ADP (3 μM) were similar in washed platelets from control and Vps33bfl/fl-ERT2 mice (n = 12 mice per genotype). (D) δ-Granule secretion was measured in the lumi-aggregometer using a luciferase assay (ATP). ATP secretion in Vps33bfl/fl and Vps33bfl/fl-ERT2 platelets (n = 12 mice per genotype) in response to 0.05 U/mL thrombin. (E) Reduced ATP secretion was observed in Vps33bfl/fl-ERT2 vs Vps33bfl/fl platelets (n = 12 mice per genotype) upon collagen stimulation. (F) ATP secretion measured by lummi-aggregometry after stimulation of washed platelets with the divalent calcium ionophore A23187 (10 μM) (n = 5 mice per genotype). (G) Tail-bleeding assay (n = 15 to 17 mice per genotype). Open circles (○) represent individual mice. Horizontal lines represent means. (H) Representative fluorescence images (DiOC6) at 2 and 4 minutes of blood perfusion (left and middle panels). Representative phase-contrast images (BF) at the end of the perfusion period (right panel). Images were obtained using a Zeiss Axiovert 200 inverted high-end microscope with a 40× objective. Scale bar, 10 µm. All values are mean ± SEM. *P < .05; **P < .01; ***P < .001. BF, bright field; MFI, mean fluorescence intensity; ns, not significant.
Characterization of Vps33bfl/fl-ERT2 platelet function. (A) VWF and P-selectin levels were measured in platelets by immunoblot analysis. β-Actin was used as a loading control. Densitometric analysis showed a reduction in VWF and P-selectin content in Vps33bfl/fl-ERT2 platelets (n = 3 mice) in comparison with Vps33bfl/fl platelets (n = 5 mice). (B) Thrombin (0.1 U/mL) induced P-selectin expression on the surface of Vps33bfl/fl-ERT2 (n = 15 mice) platelets in comparison with Vps33bfl/fl (n = 19 mice). Histogram (left) shows an IgG control (purple), P-selectin expression in controls (green and pink), and Vps33bfl/fl-ERT2 platelets (blue). (C) Representative aggregation responses to thrombin (0.05 U/mL), collagen (3 μg/mL), and ADP (3 μM) were similar in washed platelets from control and Vps33bfl/fl-ERT2 mice (n = 12 mice per genotype). (D) δ-Granule secretion was measured in the lumi-aggregometer using a luciferase assay (ATP). ATP secretion in Vps33bfl/fl and Vps33bfl/fl-ERT2 platelets (n = 12 mice per genotype) in response to 0.05 U/mL thrombin. (E) Reduced ATP secretion was observed in Vps33bfl/fl-ERT2 vs Vps33bfl/fl platelets (n = 12 mice per genotype) upon collagen stimulation. (F) ATP secretion measured by lummi-aggregometry after stimulation of washed platelets with the divalent calcium ionophore A23187 (10 μM) (n = 5 mice per genotype). (G) Tail-bleeding assay (n = 15 to 17 mice per genotype). Open circles (○) represent individual mice. Horizontal lines represent means. (H) Representative fluorescence images (DiOC6) at 2 and 4 minutes of blood perfusion (left and middle panels). Representative phase-contrast images (BF) at the end of the perfusion period (right panel). Images were obtained using a Zeiss Axiovert 200 inverted high-end microscope with a 40× objective. Scale bar, 10 µm. All values are mean ± SEM. *P < .05; **P < .01; ***P < .001. BF, bright field; MFI, mean fluorescence intensity; ns, not significant.
A bleeding diathesis, and defective adhesion and aggregate formation under flow conditions were observed in Vps33bfl/fl-ERT2 mice
In vitro assays were carried out in order to characterize the consequences of α-granule deficiency on platelet function. Expression levels of major platelet surface receptors were assessed by flow cytometry. There was no significant difference in surface receptor levels between Vps33bfl/fl and Vps33bfl/fl-ERT2 mice, other than a 20% reduction in the levels of the collagen receptor glycoprotein VI (GPVI) (Table 2). Concentration response curves for aggregation to various agonists (0.05 and 0.1 U/mL thrombin; 3 and 10 μg/mL collagen; 1, 3, and 10 μM adenosine diphosphate [ADP]) were similar to controls (n = 12) (Figure 3C and data not shown). Adenosine 5′-triphosphate (ATP) secretion from washed platelets in response to a low concentration (0.05 U/mL) of thrombin was also similar between Vps33bfl/fl-ERT2 platelets and controls (n = 12) (Figure 3D). However, a partial reduction (Vps33bfl/fl: 0.6 ± 0.1 nmol; Vps33bfl/fl-ERT2: 0.3 ± 0.1 nmol, n = 12; P < .05) in ATP secretion was observed in Vps33bfl/fl-ERT2 platelets upon collagen stimulation (Figure 3E). This reduction was due to the reduced levels of GPVI and a possible δ-granule secretion defect in Vps33bfl/fl-ERT2 mice, as secretion induced by the divalent calcium ionophore A23187, which bypasses G protein-coupled receptors, showed a significant reduction in Vps33bfl/fl-ERT2 platelets (Vps33bfl/fl: 2.5 ± 0.3 nmol; Vps33bfl/fl-ERT2: 0.7 ± 0.1 nmol, n = 5; P < .001) (Figure 3F). This data agrees with our finding of a defect in ATP secretion in 2 ARC patients (supplemental Figure 5).
Platelet surface glycoprotein expression in whole blood of Vps33bfl/fl-ERT2 mice
| Surface glycoproteins . | Vps33bfl/fl (Mean ± SEM; n = 8) . | Vps33bfl/fl-ERT2 (Mean ± SEM; n = 6) . |
|---|---|---|
| GPVI | 15.5 ± 0.7 | 12.3 ± 0.7** |
| Integrin α2 | 12.3 ± 0.7 | 11.7 ± 1.1 |
| Integrin αIIbβ3 | 140.2 ± 9.9 | 108.9 ± 13.2 |
| GP1bα | 32.1 ± 2.2 | 35.8 ± 2.9 |
| αIIbβ3 activity | 68.7 ± 5.9 | 57.8 ± 5.6 |
| Surface glycoproteins . | Vps33bfl/fl (Mean ± SEM; n = 8) . | Vps33bfl/fl-ERT2 (Mean ± SEM; n = 6) . |
|---|---|---|
| GPVI | 15.5 ± 0.7 | 12.3 ± 0.7** |
| Integrin α2 | 12.3 ± 0.7 | 11.7 ± 1.1 |
| Integrin αIIbβ3 | 140.2 ± 9.9 | 108.9 ± 13.2 |
| GP1bα | 32.1 ± 2.2 | 35.8 ± 2.9 |
| αIIbβ3 activity | 68.7 ± 5.9 | 57.8 ± 5.6 |
αIIbβ3 activity: fibrinogen binging to αIIbβ3 in response to 0.1 U/mL thrombin.
P < .01.
To investigate whether the α-granule deficiency is associated with a bleeding diathesis, a tail-bleeding assay was performed. Approximately 60% (9/15) of the Vps33bfl/fl-ERT2 mice had prolonged bleeding beyond 10 minutes (losing 3.8 ± 0.9 mg blood per gram, n = 15), whereas none of the controls bled beyond 5 minutes (losing 0.3 ± 0.1 mg blood per gram, n = 17) (P < .001) (Figure 3G). No signs of spontaneous bleeding were evident in Vps33bfl/fl-ERT2 mice. This is in line with previously published patient data showing a marked increase in life-threatening hemorrhage following organ biopsies in ARC patients.18
An in vitro shear assay allowed the study of platelet function under more physiologically relevant conditions. Anticoagulated blood was perfused over immobilized collagen at an arteriolar shear rate of 1000s−1. Under these conditions, there was a marked reduction in stable aggregate formation in Vps33bfl/fl-ERT2 mice after 4 minutes of perfusion relative to Vps33bfl/fl mice (n = 4). Specifically, small aggregates formed within the first minute of perfusion but underwent rapid embolization, such that by 4 minutes a monolayer of platelets was formed on the collagen fibers (Figure 3H). These findings are consistent with a functional platelet defect, thus emphasizing the importance of α-granules for hemostasis.
Abnormal ultrastructure of BM Vps33bfl/fl-ERT2 MKs
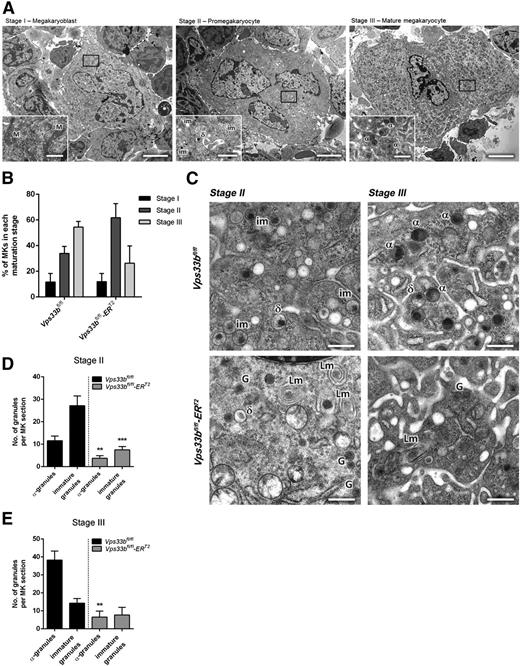
The α-granule deficiency observed in Vps33bfl/fl-ERT2 platelets was also evident in femoral BM MKs analyzed by TEM. Initially, MKs were classified into 3 categories based on cell size, nuclear morphology, presence of granules, and development of the demarcation membrane system (DMS)26 as follows: (1) stage I megakaryoblasts that were 10 to 15 μm in diameter with a single large nucleus and absence of granules; (2) stage II pro-MKs that were 15 to 30 μm in diameter with a multilobed nucleus and presence of mainly immature platelet-specific granules; and (3) stage III mature MKs that were >30 μm in diameter with a well-developed DMS and presence of mature α- and δ-granules (Figure 4A). Determination of the maturation stages per BM section (n = 32 to 44 MKs per genotype) showed that there was a nonsignificant variation for stage II and III MKs in Vps33bfl/fl-ERT2 mice (Figure 4B). However, significant differences were observed in granule formation. Two discrete populations of MKs were present in Vps33bfl/fl-ERT2 mice with over 60% of MKs lacking immature granules and α-granules. The remaining MKs are likely to have escaped CreERT2-recombinase Vps33b excision and account for the platelet population that contains normal numbers of α-granules in the Vps33bfl/fl-ERT2 mice. In MKs devoid of α-granules, the presence of small α-granule–like structures was noted that are similar to those observed in Vps33bfl/fl-ERT2 platelets (Figure 4C, bottom panels). Such granules were absent in controls (Figure 4C, top panels). Moreover, MKs from Vps33bfl/fl-ERT2 mice had a large number of lamellar structures that were located either close to the nucleus or to the periphery of the MK (Figure 4C). Quantification of the number of granules in stage II MKs revealed a marked decrease in α-granules (3.7 ± 1.1 per MK section) and immature granules (7.5 ± 1.5 per MK section) in Vps33bfl/fl-ERT2 mice compared with controls (11.5 ± 2.1 and 27.2 ± 4.4 per MK section, respectively) (P < .01) (Figure 4D). Similarly, the number of α-granules in stage III MKs showed a dramatic decrease in Vps33bfl/fl-ERT2 (6.6 ± 3.3 per MK section) compared with Vps33bfl/fl mice (38.3 ± 5.0 per MK section) (P < .01) (Figure 4E).
Abnormal ultrastructure of femoral BM Vps33bfl/fl-ERT2 MKs. (A) Representative transmission electron micrographs of femoral BM sections showing the classification of MKs in 3 main maturation stages. Scale bars, main image 5 μm; inset 1 μm. (B) Quantification of the percentage of MKs present per maturation stage in Vps33bfl/fl and Vps33bfl/fl-ERT2 mice (n = 32 to 44 MKs analyzed in 3 mice per genotype). (C) Vps33bfl/fl MKs showing nice distribution of granules in maturation stages II and III (top panels), whereas Vps33bfl/fl-ERT2 MKs were devoid of morphologically distinct α-granules but were abundant of small α-granule–like structures (bottom panels). Clusters of lamellar structures were also evident in Vps33bfl/fl-ERT2 MKs. Scale bar, 0.5 μm. (D-E) Quantification of α-granules and immature granules at maturation stage II (D) and III (E) reveals a marked decrease in their numbers in Vps33bfl/fl-ERT2 mice when compared with controls (n = 32 to 44 MKs analyzed in 3 mice per genotype). All values are mean ± SEM. **P < .01; ***P < .001. α, α-granule; δ, δ-granule; G, α-granule-like structure; im, immature granule; Lm, lamellar structure; M, mitochondrion.
Abnormal ultrastructure of femoral BM Vps33bfl/fl-ERT2 MKs. (A) Representative transmission electron micrographs of femoral BM sections showing the classification of MKs in 3 main maturation stages. Scale bars, main image 5 μm; inset 1 μm. (B) Quantification of the percentage of MKs present per maturation stage in Vps33bfl/fl and Vps33bfl/fl-ERT2 mice (n = 32 to 44 MKs analyzed in 3 mice per genotype). (C) Vps33bfl/fl MKs showing nice distribution of granules in maturation stages II and III (top panels), whereas Vps33bfl/fl-ERT2 MKs were devoid of morphologically distinct α-granules but were abundant of small α-granule–like structures (bottom panels). Clusters of lamellar structures were also evident in Vps33bfl/fl-ERT2 MKs. Scale bar, 0.5 μm. (D-E) Quantification of α-granules and immature granules at maturation stage II (D) and III (E) reveals a marked decrease in their numbers in Vps33bfl/fl-ERT2 mice when compared with controls (n = 32 to 44 MKs analyzed in 3 mice per genotype). All values are mean ± SEM. **P < .01; ***P < .001. α, α-granule; δ, δ-granule; G, α-granule-like structure; im, immature granule; Lm, lamellar structure; M, mitochondrion.
Investigation of intermediate steps in α-granule formation in cultured MKs
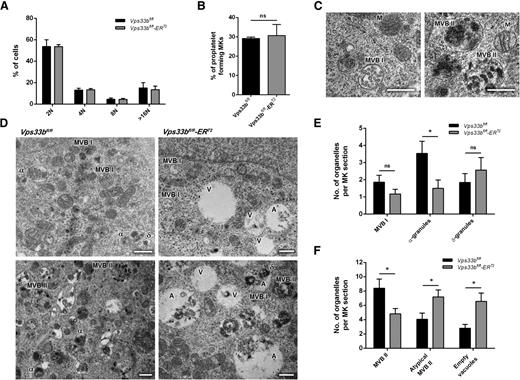
The ultrastructural abnormalities in femoral BM Vps33bfl/fl-ERT2 MKs are consistent with defects in the intermediate steps of α-granule biogenesis. In order to investigate this in further detail, MKs were cultured from BM-derived hematopoietic stem cells for 5 days in the presence of stem cell factor and thrombopoietin. VPS33B deficiency had no significant effect on MK maturation, as determined by measurement of ploidy between Vps33bfl/fl and Vps33bfl/fl-ERT2 MKs (Figure 5A). Quantification showed that Vps33bfl/fl-ERT2 MKs were able to form proplatelets similar to Vps33bfl/fl MKs (Figure 5B and supplemental Figure 6A).
Characterization of Vps33bfl/fl-ERT2 MKs in primary culture. (A) Distribution of Vps33bfl/fl and Vps33bfl/fl-ERT2 BM-derived MKs ploidy after 5 days in culture. The percentage of cells with 2N to 128N ploidy was quantified by propidium iodide staining and flow cytometry (n = 5 mice per genotype). Mean ± SD. (B) Proplatelet formation was unaltered in Vps33bfl/fl-ERT2 MKs (n = 3 mice per genotype). Mean ± SD. (C) Transmission electron micrographs of BM-derived MKs showing representative images of MVB I (left) and MVB II (right) in control MKs. Scale bar, 0.5 μm. (D) Representative transmission electron micrographs of BM-derived MKs. Vps33bfl/fl MKs had normal α- and δ-granules, whereas MBV I and MBV II were also present (left panels). Note the presence of large vacuolar structures in Vps33bfl/fl-ERT2 MKs (right panels). Scale bar, 0.5 μm. (E-F) Quantification of organelles present in MK sections. Number of MVB I, α-, and δ-granules (E), and number of classical and atypical MVB II and vacuoles (F) per MK section. Twenty to 27 MKs imaged per genotype, 4 to 5 fields of view (4.98 × 3.32 μm) per MK taken at a magnification of ×30 000. Mean ± SEM. *P < .05. A, atypical MVB II; α, α-granule; δ, δ-granule; M, mitochondrion; ns, not significant; V, empty vacuole.
Characterization of Vps33bfl/fl-ERT2 MKs in primary culture. (A) Distribution of Vps33bfl/fl and Vps33bfl/fl-ERT2 BM-derived MKs ploidy after 5 days in culture. The percentage of cells with 2N to 128N ploidy was quantified by propidium iodide staining and flow cytometry (n = 5 mice per genotype). Mean ± SD. (B) Proplatelet formation was unaltered in Vps33bfl/fl-ERT2 MKs (n = 3 mice per genotype). Mean ± SD. (C) Transmission electron micrographs of BM-derived MKs showing representative images of MVB I (left) and MVB II (right) in control MKs. Scale bar, 0.5 μm. (D) Representative transmission electron micrographs of BM-derived MKs. Vps33bfl/fl MKs had normal α- and δ-granules, whereas MBV I and MBV II were also present (left panels). Note the presence of large vacuolar structures in Vps33bfl/fl-ERT2 MKs (right panels). Scale bar, 0.5 μm. (E-F) Quantification of organelles present in MK sections. Number of MVB I, α-, and δ-granules (E), and number of classical and atypical MVB II and vacuoles (F) per MK section. Twenty to 27 MKs imaged per genotype, 4 to 5 fields of view (4.98 × 3.32 μm) per MK taken at a magnification of ×30 000. Mean ± SEM. *P < .05. A, atypical MVB II; α, α-granule; δ, δ-granule; M, mitochondrion; ns, not significant; V, empty vacuole.
Examination of cultured MKs by TEM enabled further investigation of the earlier events in α-granule biogenesis. It has been shown previously that MVBs were much more prominent in cultured MKs than in BM MKs and platelets.8 Two types of MVBs could be distinguished based on their internal morphology: (1) MVB I that contain abundant intraluminal vesicles (Figure 5C, left panel); and (2) MVB II that contain electron-dense material together with intraluminal vesicles (Figure 5C, right panel). During normal MK development, MVB I undergo a gradual transition into MVB II and subsequently into α-granules.8 In Vps33bfl/fl mice, MKs contained α- and δ-granules along with both types of MVBs (Figure 5D-F). In contrast, Vps33bfl/fl-ERT2 MKs showed a significant reduction in α-granules (Vps33bfl/fl: 3.5 ± 0.7 per MK section; Vps33bfl/fl-ERT2: 1.5 ± 0.5 per MK section; P < .05) but not δ-granules (Vps33bfl/fl: 1.8 ± 0.5 per MK section; Vps33bfl/fl-ERT2: 2.6 ± 0.7 per MK section) (Figure 5D-E). Both types of classical MVB I and II were identified; however, there was a significant decrease in the MVB II number in cultured MKs from Vps33bfl/fl-ERT2 mice (Vps33bfl/fl: 8.4 ± 1.3 per MK section; Vps33bfl/fl-ERT2: 4.8 ± 0.8 per MK section; P < .05). Interestingly, there was an accumulation of large vacuolar structures clearly evident in Vps33bfl/fl-ERT2 MKs (Figure 5D,F). Two types of vacuolar structures were identified, which were both on average larger in size than MVB II: (1) empty vacuoles; and (2) vacuoles partly filled with electron-dense material referred to as atypical MVB II (Figure 5D, right panels). Both types of vacuoles were significantly increased in numbers in Vps33bfl/fl-ERT2 MKs (vacuoles: 6.6 ± 1.2 per MK section; atypical: 7.2 ± 1.0 per MK section) when compared with Vps33bfl/fl MKs (vacuoles: 2.8 ± 0.5 per MK section; atypical: 4.1 ± 0.9 per MK section) (P < .05) (Figure 5F).
Abnormalities in the trafficking of α-granule cargo proteins in Vps33bfl/fl-ERT2 mice
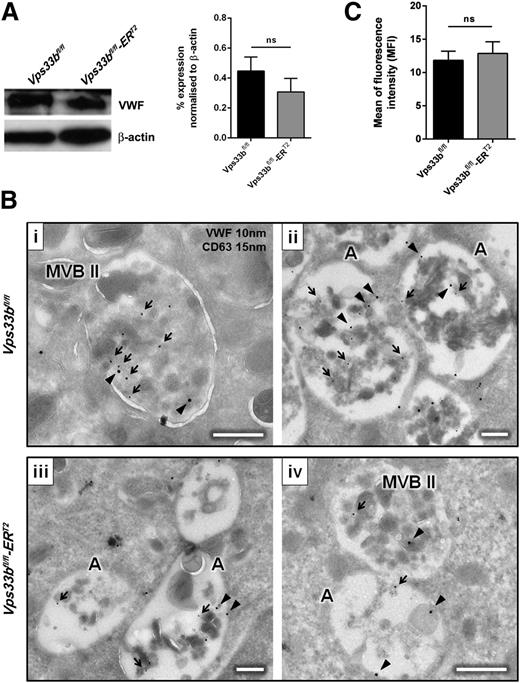
In an attempt to test whether trafficking of endogenously synthesized proteins was abnormal in the Vps33bfl/fl-ERT2 mice, the localization of VWF in MKs was assessed. Immunoblotting had shown that Vps33bfl/fl-ERT2 MKs synthesize VWF at levels equivalent to controls (Figure 6A), excluding a defect in protein production. More detailed examination by immunogold labeling on cryosections of cultured MKs from Vps33bfl/fl mice showed that VWF was detected mainly within MVB II and at lower levels in the dense material of the atypical MVB II. The tetraspanin protein CD63 was equally found in classical and atypical MVB II (Figure 6Bi-ii). In contrast, there was a significant reduction in the number of VWF and CD63 gold particles present in MVB II from Vps33bfl/fl-ERT2 MKs (Figure 6Biii-iv). The ratio of VWF gold particles per MVB II between Vps33bfl/fl and Vps33bfl/fl-ERT2 MKs was 3 to 1, whereas with CD63 it was 1 to 0.6 (P < .05). Then, we determined the percentage of MVB II, atypical MVB II, and vacuoles that showed VWF, CD63, or VWF/CD63 labeling (Table 3). A marked reduction in the percentage of VWF- and VWF/CD63-labeled MVB II was observed in Vps33bfl/fl-ERT2 mice when compared with controls, whereas CD63-labeled MVB II were significantly increased. In addition, there was a twofold increase in unlabeled MVB II and a nonsignificant trend of increase in unlabeled vacuolar structures in Vps33bfl/fl-ERT2 MKs (Table 3).
Abnormal trafficking of VWF in Vps33bfl/fl-ERT2 mice. (A) VWF levels were measured in MKs by immunoblot (left panel) and densitometry (right panel) using a rabbit polyclonal antibody anti-VWF (H-300) (1:1000), and a goat anti-rabbit and a goat anti-mouse horseradish peroxidase-conjugate secondary antibody (1:1000). β-Actin was used as a loading control (n = 3 mice per genotype). (B) Immunogold labeling (IEM) using the Tokuyasu method for double labeling of VWF and CD63 (i-iv) in MKs from Vps33bfl/fl and Vps33bfl/fl-ERT2 mice. Black arrowheads, VWF 10 nm gold particles; black arrows, CD63 15 nm gold particles. Scale bar, 250 nm. A total of 15 MKs were imaged per genotype, 4 to 5 fields of view (4.98 × 3.32 μm) per MK taken at a magnification of ×30 000. (C) Fibrinogen uptake in cultured MKs after incubation with 488-fibrinogen for 2 hours. Mean ± SEM. A, atypical MVB II, MFI, mean fluorescence intensity; ns, not significant.
Abnormal trafficking of VWF in Vps33bfl/fl-ERT2 mice. (A) VWF levels were measured in MKs by immunoblot (left panel) and densitometry (right panel) using a rabbit polyclonal antibody anti-VWF (H-300) (1:1000), and a goat anti-rabbit and a goat anti-mouse horseradish peroxidase-conjugate secondary antibody (1:1000). β-Actin was used as a loading control (n = 3 mice per genotype). (B) Immunogold labeling (IEM) using the Tokuyasu method for double labeling of VWF and CD63 (i-iv) in MKs from Vps33bfl/fl and Vps33bfl/fl-ERT2 mice. Black arrowheads, VWF 10 nm gold particles; black arrows, CD63 15 nm gold particles. Scale bar, 250 nm. A total of 15 MKs were imaged per genotype, 4 to 5 fields of view (4.98 × 3.32 μm) per MK taken at a magnification of ×30 000. (C) Fibrinogen uptake in cultured MKs after incubation with 488-fibrinogen for 2 hours. Mean ± SEM. A, atypical MVB II, MFI, mean fluorescence intensity; ns, not significant.
Percentages of MVB II, atypical MVB II, and vacuoles labeled with platelet-specific markers in cultured MKs
| Proteins labeled . | Vps33bfl/fl . | Vps33bfl/fl-ERT2 . | ||||
|---|---|---|---|---|---|---|
| MVB II . | Atypical MVB II . | Empty vacuoles . | MVB II . | Atypical MVB II . | Empty vacuoles . | |
| VWF | 35.5 ± 6.5 | 36.8 ± 12.3 | 17.9 ± 9.7 | 17.3 ± 5.2* | 29.5 ± 7.2 | 10.9 ± 4.5 |
| CD63 | 6.8 ± 2.3 | 14.8 ± 6.2 | 27.4 ± 15.3 | 19.5 ± 4.2* | 19.4 ± 5.9 | 27.8 ± 4.7 |
| VWF/CD63 | 35.7 ± 5.5 | 28.9 ± 10.7 | 28.6 ± 13.9 | 19.6 ± 5.3* | 19.1 ± 5.8 | 19.3 ± 10.2 |
| Unlabeled | 22.0 ± 6.1 | 19.5 ± 9.8 | 26.2 ± 15.4 | 43.5 ± 7.6* | 32.0 ± 6.8 | 42.0 ± 7.0 |
| Proteins labeled . | Vps33bfl/fl . | Vps33bfl/fl-ERT2 . | ||||
|---|---|---|---|---|---|---|
| MVB II . | Atypical MVB II . | Empty vacuoles . | MVB II . | Atypical MVB II . | Empty vacuoles . | |
| VWF | 35.5 ± 6.5 | 36.8 ± 12.3 | 17.9 ± 9.7 | 17.3 ± 5.2* | 29.5 ± 7.2 | 10.9 ± 4.5 |
| CD63 | 6.8 ± 2.3 | 14.8 ± 6.2 | 27.4 ± 15.3 | 19.5 ± 4.2* | 19.4 ± 5.9 | 27.8 ± 4.7 |
| VWF/CD63 | 35.7 ± 5.5 | 28.9 ± 10.7 | 28.6 ± 13.9 | 19.6 ± 5.3* | 19.1 ± 5.8 | 19.3 ± 10.2 |
| Unlabeled | 22.0 ± 6.1 | 19.5 ± 9.8 | 26.2 ± 15.4 | 43.5 ± 7.6* | 32.0 ± 6.8 | 42.0 ± 7.0 |
MVB II, atypical MVB II, and vacuoles were counted over cryosections of cultured MKs after immunolabeling with VWF and CD63. The data are expressed as percentages of a structure that contains gold particles over the total number of the selected structure. Fifteen MKs imaged per genotype, 4 to 5 fields of view per MK (4.98 × 3.32 μm). All values are mean ± SEM.
P < .05.
We also investigated if the lack of VPS33B would affect endocytosis. Cultured MKs were incubated with fluorescently-labeled fibrinogen for 2 hours and the level of uptake was assessed by flow cytometry. There was no difference in fibrinogen uptake between control and Vps33bfl/fl-ERT2–derived MKs (Figure 6C). This suggested that sorting of newly synthesized (and not endocytosed) proteins into α-granule progenitor MVBs was the most likely reason for the α-granule biogenesis defect.
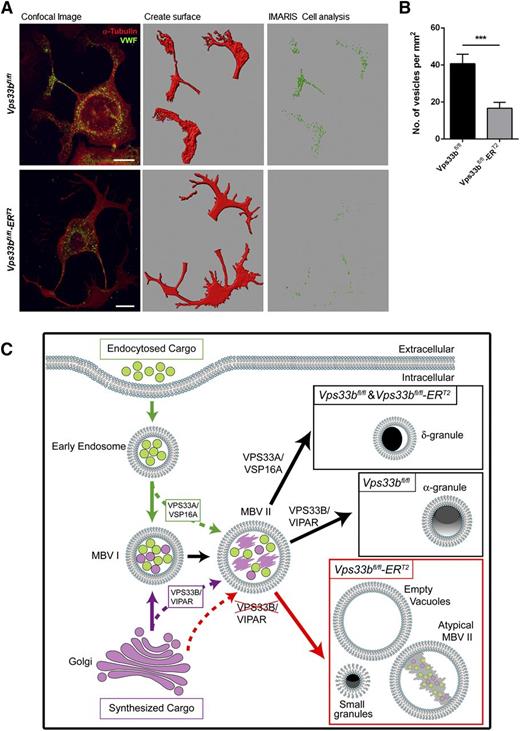
We then asked the question whether VWF can be trafficked to proplatelet extensions during proplatelet formation despite the absence of normal-sized α-granules. To this end, cultured MKs were spread on fibrinogen for 6 hours in order to give sufficient time to generate proplatelets. Confocal immunofluorescence microscopy and analysis with ImarisCell software revealed that trafficking of VWF-containing vesicles into proplatelet extensions was reduced by ∼60% in Vps33bfl/fl-ERT2 (16.6 ± 3.2 vesicles per mm2) compared with Vps33bfl/fl MKs (40.7 ± 5.1 vesicles per mm2) (P < .001) (Figure 7A-B). This was in agreement with the levels of VWF present in platelets of Vps33bfl/fl-ERT2 mice. The tubulin and actin organization were unaffected in Vps33bfl/fl-ERT2 MKs, providing evidence against a defect in vesicle trafficking to proplatelets due to cytoskeletal abnormalities (supplemental Figure 6A-B). Taken together, the results above are in agreement with a defect in trafficking of cargo proteins such as VWF to MVB II in Vps33bfl/fl-ERT2 MKs, which results in abnormal MVB maturation leading to defective α-granule biogenesis.
Trafficking of VWF to proplatelet extensions. (A) VWF distribution (green) during proplatelet formation. Tubulin was used to stain the cytoskeleton (red). Confocal immunofluorescence images (left panels) were analyzed with ImarisCell, an analytical tool by Bitplane that quantifies cellular morphology. Different steps in image analysis are shown here (middle and right panels). Scale bar, 30 μm. (B) Quantification of the number of VWF-containing vesicles from confocal immunofluorescence images by the use of ImarisCell. Results are shown as number of vesicles per mm2 of proplatelet area (n = 30 MKs imaged from 3 mice per genotype). Mean ± SEM; ***P < .001. (C) Suggested model for the function of VPS33B homologs in platelet granule biogenesis. Early endosomes are formed by endocytosis of cargo and following maturation they lead to MVB I (green arrows). MVB I communicate with the Golgi apparatus receiving vesicles with newly synthesized cargo (purple arrow). MVB I undergo further maturation to MVB II that may receive additional cargo for sorting (dotted green and purple arrows). VPS33A and its interacting partner VPS16A are required for sorting of proteins from endosomes into maturing MVB II leading to the formation of δ-granules. On the other hand, VPS33B in complex with VIPAR is likely to be responsible for sorting of cargo from the trans-Golgi network to α-granule–destined MVBs and subsequently promoting α-granule formation. VPS33B deficiency results in a defect in trafficking of some cargo proteins to MVB II (dotted red arrow) resulting in abnormal MVB maturation and defective α-granule biogenesis (red arrow). Accumulation of large vacuolar structures and the presence of small granules are characteristics of those MKs. A possible role of VPS33B in the sorting of some δ-granule proteins cannot be ruled out.
Trafficking of VWF to proplatelet extensions. (A) VWF distribution (green) during proplatelet formation. Tubulin was used to stain the cytoskeleton (red). Confocal immunofluorescence images (left panels) were analyzed with ImarisCell, an analytical tool by Bitplane that quantifies cellular morphology. Different steps in image analysis are shown here (middle and right panels). Scale bar, 30 μm. (B) Quantification of the number of VWF-containing vesicles from confocal immunofluorescence images by the use of ImarisCell. Results are shown as number of vesicles per mm2 of proplatelet area (n = 30 MKs imaged from 3 mice per genotype). Mean ± SEM; ***P < .001. (C) Suggested model for the function of VPS33B homologs in platelet granule biogenesis. Early endosomes are formed by endocytosis of cargo and following maturation they lead to MVB I (green arrows). MVB I communicate with the Golgi apparatus receiving vesicles with newly synthesized cargo (purple arrow). MVB I undergo further maturation to MVB II that may receive additional cargo for sorting (dotted green and purple arrows). VPS33A and its interacting partner VPS16A are required for sorting of proteins from endosomes into maturing MVB II leading to the formation of δ-granules. On the other hand, VPS33B in complex with VIPAR is likely to be responsible for sorting of cargo from the trans-Golgi network to α-granule–destined MVBs and subsequently promoting α-granule formation. VPS33B deficiency results in a defect in trafficking of some cargo proteins to MVB II (dotted red arrow) resulting in abnormal MVB maturation and defective α-granule biogenesis (red arrow). Accumulation of large vacuolar structures and the presence of small granules are characteristics of those MKs. A possible role of VPS33B in the sorting of some δ-granule proteins cannot be ruled out.
Discussion
Inherited α-granule storage pool disorders in humans are rare, heterogeneous, and usually associated with a variable tendency for bleeding. Agranular gray-appearing platelets are a cardinal feature of both GPS27,28 and ARC syndromes23 that are characterized by a severe reduction in α-granule number and protein content. The current study presents the first mouse model for ARC syndrome, Vps33bfl/fl-ERT2, where Vps33b is ubiquitously excised post-development. The Vps33bfl/fl-ERT2 mouse model was created due to embryonic lethality of the ubiquitous Vps33bfl/fl-PGKCre mouse at E7.5 (data not included) and because of the relatively mild platelet phenotype of the Vps33bfl/fl-PF4Cre mouse, which may reflect a role for VPS33B prior to Cre-expression (data not included). It should be noted here that the tamoxifen induction was timed to demonstrate the platelet rather than a global phenotype. This is achieved because of the short half-life of platelets in the circulation (5 days) relative to the much longer half-life of other VPS33B-expressing cells.
In an attempt to understand the molecular mechanism of VPS33B function during α-granule formation, the Vps33bfl/fl-ERT2 mouse was validated as a model of ARC syndrome. Whole-mount and transmission EM confirmed the reduction in the number of α-granules but not δ-granules in Vps33bfl/fl-ERT2 mouse platelets in line with the human phenotype. A variable efficiency of deletion after tamoxifen administration may account for the presence of a small subpopulation of Vps33bfl/fl-ERT2 platelets with normal numbers of α-granules.29 Interestingly, small VWF-containing granules were observed in Vps33bfl/fl-ERT2 mouse platelets that may correspond to abnormally formed α-granules. Ultrastructural analysis of platelets from 2 ARC patients with VPS33B mutations and an ARC patient with a VIPAS39 mutation revealed the presence of similar, small abnormal granules in ARC platelets (this study). Small granules have also been reported in patients with GPS.30 The presence of normal levels of α-granule proteins in MKs but reduced levels and mislocalization in platelets from Vps33bfl/fl-ERT2 mice is indicative of abnormal protein sorting rather than defective protein synthesis.
Vps33bfl/fl-ERT2 platelets contained normal levels of the major glycoprotein surface receptors and underwent a similar pattern of aggregation to that in controls in response to the major platelet agonists. This is in accordance with data from the majority of ARC patients, although in a few cases, the absence of secondary wave was observed in response to collagen and ADP.23,24 A qualitative platelet function defect was demonstrated in Vps33bfl/fl-ERT2 mice by the reduced levels of GPVI on the plasma membrane. A similar reduction in GPVI levels was observed in MKs from Vps33bfl/fl-ERT2 mice, suggesting that it occurs at the level of the MK (data not shown). We also identified a δ-granule secretion defect in Vps33bfl/fl-ERT2 mice, even though the number of δ-granules was not altered. A reduction in δ-granule secretion was also observed in a VPS33B and a VIPAR deficient patient. It is unclear if the defect is due to abnormal sorting of cargo proteins in MKs or abnormal fusion of δ-granules to the plasma membrane in the mature platelet.
In addition to the defects in platelet function, platelet adhesion and aggregate formation were reduced under flow conditions in Vps33bfl/fl-ERT2 mice, consistent with a critical role of platelet α-granules in platelet aggregation at intermediate and high rates of shear. This underlines the importance of key adhesion molecules, such as fibrinogen and VWF, that reside in α-granules in building stable thrombi at high shear rates and their contribution to the arrest of bleeding after trauma or surgical procedures. Indeed, it has been previously reported that patients with ARC syndrome have an increased risk of hemorrhage, especially when they are challenged, that may lead to morbidity and mortality.18 Our studies show a similar bleeding diathesis in a tail-bleeding assay in Vps33bfl/fl-ERT2 mice; however, spontaneous bleeding was not observed.
Our studies on BM native and cultured MKs revealed normal MK maturation and proplatelet formation in Vps33bfl/fl-ERT2 mice in comparison with controls. This indicates that VPS33B is not involved in endomitosis, the formation of DMS, and in the terminal steps of platelet production. Quantitative TEM analysis of native BM Vps33bfl/fl-ERT2 MKs showed a marked reduction in immature and mature α-granules, and the accumulation of lamellar structures. Multilamellar bodies are detected by EM in various tissues (lung type II alveolar cells and skin) under physiological conditions but are not normally seen in the MKs. They are lipid-protein complexes (which can be identified by light microscopy as lipofuscin granules) that are variable in size and may also contain apolipoproteins and lytic enzymes, and have an acidic pH.31 Multilamellar bodies also accumulate in various pathological conditions, such as atherosclerosis, lysosomal storage, and trafficking disorders (eg, Tay–Sachs, Fabry, and Niemann–Pick diseases) and in wound healing.31-33 These lamellar structures are likely to contain accumulation of missorted proteins and lipids. In ARC patients, the accumulation of lipofuscin has been previously reported in neonatal liver biopsies and granules containing lamellar and vesicular aggregates were detected in skin fibroblasts, whereas ultrastructural examination of the skin cornified cells revealed impaired secretion of lamellar granules.34,35
The use of MKs cultured from BM-derived hematopoietic stem cells allowed the study of intermediate steps in α-granule biogenesis. Quantitative TEM analysis of BM-derived MKs in culture showed a reduction in α-granules and MVB II, and the presence of large vacuolar structures in Vps33bfl/fl-ERT2 mice. Apart from empty vacuoles, an increased number of atypical MVB II was observed. The latter structures contained aggregates of vesicles and enveloping membranes partly filled with amorphous electron dense material and were labeled for the α-granule cargo proteins VWF and CD63. There was a marked reduction of VWF and VWF/CD63-positive MVB II and an increase of CD63-positive MVB II in the MKs derived from Vps33bfl/fl-ERT2 mice, suggesting a defective trafficking of secreted cargo proteins into MVB II. These abnormal MVBs, instead of progressing to α-granules, could be targeted for lysosomal degradation through the autophagic pathway resulting in the presence of lamellar structures observed in the native BM MKs from Vps33bfl/fl-ERT2 mice. Thus, it appears that VPS33B plays a key role in the synthesis of α-granules at the level of maturing MVB II. A related observation was made in Drosophila where a mutation in the full-of-bacteria gene, which encodes Drosophila Vps16B (a homolog of human VIPAR and the partner of VPS33B), is required for phagosome maturation during immune defense.36
This work and evidence from previous studies demonstrate that mutations in VPS33B result in platelet α-granule (but not δ-granule) formation defect in humans and mice.24,25 VPS33B is a homolog of yeast Vps33, which forms a part of large protein complexes homotypic protein sorting (HOPS) and class C core vacuole/endosome tethering (CORVET), involved at different stages of vacuolar biogenesis. In mammalian cells, HOPS has a role in late endosome and lysosome formation and also autophagosome biogenesis, whereas CORVET acts at the early endosome stages of protein sorting.37,38 In multicellular organisms, two Vps33 homologs are present, VPS33A and VPS33B. There are several lines of evidence to suggest that VPS33A and VPS33B have evolved different functions in metazoans. VPS33A is a major constituent of HOPS and CORVET,39 and the mouse Vps33a buff mutation causes Hermansky–Pudlak syndrome phenotype with defective melanosome and platelet δ-granule maturation but normal α-granules.40 VPS33B co-localizes with the trans-Golgi network and interacts with Rab11a, which regulates recycling endosome and trans-Golgi trafficking.20,24,25
The observation that VPS33B-deficient platelets lack α-granules but contain δ-granules, whereas Vps33a mutant platelets are devoid of δ-granules but contain α-granules suggests that VPS33A and VPS33B are involved in two distinct mechanisms by which these granules develop from MKs. It has been shown that in maturing BM MKs, δ-granule cargo originates from small vesicles budding from early endosome-associated tubules.41,42 In contrast, platelet α-granules receive their cargo from the endocytic pathway as well as from the trans-Golgi network. Thus, it could be proposed that VPS33A, as part of HOPS or CORVET, is important for the sorting of proteins from endosomes into maturing MVBs leading to the formation of δ-granules (Figure 7C). On the other hand, VPS33B in complex with VIPAR is likely to be responsible for sorting of some cargoes from the trans-Golgi network to α-granule–destined MVBs, and subsequently promoting their maturation (Figure 7C). Identification of a δ-granule secretion defect in Vps33bfl/fl-ERT2 mice suggests an additional function of VPS33B either in sorting some of the δ-granule cargo proteins or in δ-granule to plasma membrane fusion. Further work is required to accurately delineate other proteins involved in these pathways.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Alice Pollitt for useful discussions and preparation of the trafficking model in Figure 7C and Paul Saftig for his generous gift of the CD63 antibody.
This study was supported by a grant from the British Heart Foundation Programme (RG/09/007).
Authorship
Contribution: D.B. designed experiments, performed research, and wrote the manuscript; H.S. designed experiments and performed research; D.B., J.J.B., and I.J.W. performed EM; B.B. and J.H. managed the mouse colony, performed genotyping, and assisted with experiments; N.J. and F.R.-L. performed the mouse immunophenotyping; R.B., G.A., and A.D.M. recruited the patients; S.G.T. carried out the tail-bleeding test, and designed and supported the imaging studies; P.G. and S.P.W. conceived the study, obtained the funding, and supervised the experiments; and all authors revised the manuscript.
Conflict-of-interest disclosure: J.J.B. and I.J.W. were supported by the Medical Research Council Laboratory for Molecular Cell Biology. S.P.W. is a British Heart Foundation Chair (CH/03/003). P.G. is a Wellcome Trust Senior Research Fellow in Clinical Sciences (MT095662MA). The remaining authors declare no competing financial interests.
Correspondence: Paul Gissen, MRC Laboratory for Molecular Cell Biology, University College London, Gower St, London WC1E 6BT, United Kingdom; e-mail: p.gissen@ucl.ac.uk; and Steve P. Watson, Centre for Cardiovascular Sciences, University of Birmingham, Edgbaston, Birmingham B15 2TT, United Kingdom; e-mail: s.p.watson@bham.ac.uk.
References
Author notes
D.B. and H.S. contributed equally to this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal