Congenital defects in platelet granule formation, although unusual, have been instrumental in providing a glimpse into the mechanisms of granule biogenesis. In this issue of Blood, Bem et al now generate and characterize a murine model of arthrogryposis, renal dysfunction, and cholestasis (ARC) syndrome with an inducible deficiency in VPS33B that sheds new light on the molecular pathways involved in α-granule biogenesis.1
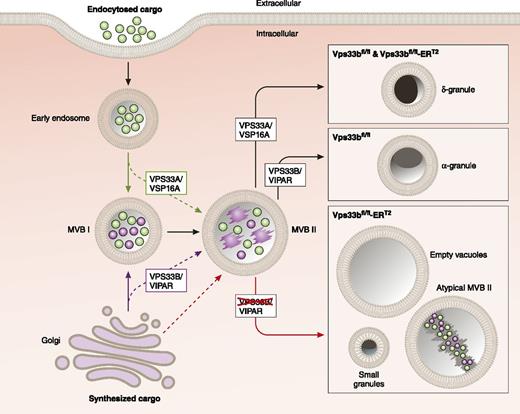
Role of VPS33B in α-granule formation. Proteins are delivered to MVB I through the trans-Golgi network via the synthetic pathway and through the plasma membrane via the endocytotic pathway. VPS33B in complex with VIPAR (VPS16B) mediates maturation of α-granules during the transition from MVB I to MVB II, and potentially during the delivery of cargos directly from the Golgi to MVB II. VPS33A and VSP16A function in dense granule maturation. The figure is adapted from Figure 7C in the article by Bem et al that begins on page 133, by professional illustrator Patrick Lane, ScEYEnce Studios.
Role of VPS33B in α-granule formation. Proteins are delivered to MVB I through the trans-Golgi network via the synthetic pathway and through the plasma membrane via the endocytotic pathway. VPS33B in complex with VIPAR (VPS16B) mediates maturation of α-granules during the transition from MVB I to MVB II, and potentially during the delivery of cargos directly from the Golgi to MVB II. VPS33A and VSP16A function in dense granule maturation. The figure is adapted from Figure 7C in the article by Bem et al that begins on page 133, by professional illustrator Patrick Lane, ScEYEnce Studios.
Much of what we have learned about platelet granule biogenesis has been derived from the evaluation of patients with inherited platelet granule deficiencies. The detailed evaluation of megakaryocytes (MKs) and platelets from patients with Hermansky–Pudlak syndrome2 or Chediak–Higashi syndrome,3 and the elucidation of the causative genes in these diseases has been critical in dissecting the membrane trafficking pathways that direct dense granule formation. More recently, the study of MKs and platelets from patients with gray platelet syndrome4 and the elucidation of affected genes has been important in understanding how α-granules are formed. Arguably, these experiments of nature have been the most revealing source of information to date for investigating platelet granulogenesis.
In 1990, another such experiment of nature was described as part of a syndrome that includes ARC syndrome.5 Patients with ARC syndrome die in infancy owing to metabolic complications or bleeding. Evaluation of their platelets reveals that they have diminutive or deficient α-granules. In contrast, their dense granules are normal or even increased in number.6 ARC syndrome was found to be caused by mutations in genes that encode for VPS33B or VIPAR (aka VPS16B),7 which form a complex that functions in membrane trafficking.
As the number of actors that contribute to platelet granule biogenesis increases (eg, BLOC proteins, AP3, NBEAL2, VPS33B, VPS16B, lysosomal-trafficking regulator, and Rabs), there is a growing need to understand how these proteins are assembled into membrane trafficking pathways responsible for granulogenesis. Such pathways should explain how the MK and platelet achieves sorting of dense granules from α-granules and the proper packaging of cargo proteins into granules. Many of the strategies used to study these membrane trafficking pathways in nucleated cells, such as gene knockdown and expression of fluorescent protein chimeras, cannot be performed in the anucleate platelet. Furthermore, it is still challenging to replicate and manipulate the complex transformation of MKs to fully functioning platelets with normal granules in vitro. It is also important to characterize the physiological consequences of granule deficiencies. For these reasons, murine models have loomed large in the characterization of platelet granule defects.
Dr Paul Gissen from the University of Birmingham, working with the laboratory of Dr Steven Watson, have now engineered and characterized a mouse with a tamoxifen-inducible deficiency in VPS33B. Dr Gissen was the first to demonstrate that mutations in VPS33B cause ARC syndrome.7 As with patients with ARC syndrome, VPS33B-deficient mice had a bleeding diathesis and platelets with markedly decreased numbers of α-granules but normal numbers of dense granules. No defects were detected in platelet aggregation. However, there was a significant impairment of dense granule release despite lack of evidence for a dense granule storage defect. Platelet adhesion under flow was substantially impaired. The degree to which impaired dense granule secretion vs α-granule deficiency contributed to the hemostatic defects observed in VPS33B-deficient mice is difficult to determine. Overall, however, the platelet abnormalities in the inducible VPS33B-deficient mice were similar to those found in humans.
In addition to the platelet function phenotyping, what distinguished this work from previous studies of human samples from ARC patients6 were the detailed studies of the ultrastructure of VPS33B-deficient MKs. The authors used quantitative analyses of micrographs in which they counted membrane structures implicated in membrane trafficking to generate new hypotheses about the molecular pathways responsible for α-granule biogenesis. What were the structures of interest? α-Granule precursors initially bud off the trans-Golgi network (see figure). They are then incorporated into specialized late endosomal structures termed multivesicular bodies (MVBs). In MKs, two types of MVBs have been described (see figure). MVB I's have undergone internal vesiculation but do not possess electron dense material. MVB II's are matured such that the granule contents are condensed and electron dense on electron microscopy.8 Evaluation of MKs demonstrated that although VPS33B deficiency does not impair protein synthesis, endomitosis, or even the formation of MVB I, it blocks the formation of MVB II. In addition, VPS33B-deficient MKs demonstrated abnormal structures, termed multilamellar bodies, consisting of multiple circumferential membranes, which can result from mis-sorting of membranes during trafficking.
The authors also evaluated protein trafficking using von Willebrand factor (VWF) as an archetype cargo protein. They showed that although control and VPS33B-deficient MKs synthesized equal concentrations of VWF, trafficking of VWF was very different between them. VWF localized to the MVB I in MKs of both genotypes. Yet very little VWF in VPS33B-deficient MKs made it to the MVB II. In contrast, a significantly higher percentage of MVB II from VPS33B-deficient MKs stained for the membrane-bound tetraspanin protein, CD63. This observation suggests that the defect in protein trafficking was selective for cargo proteins and did not impair trafficking of granule membrane proteins. The authors also demonstrated a marked reduction in the delivery of VWF-containing granules to proplatelet extensions. Taken together, these results indicate that VPS33B serves an essential role in trafficking cargo proteins from MVB I to MVB II (see figure) and show that if the cargo does not make it to the MVB II, it does not get incorporated into platelets.
Understanding α-granule formation will be important for many reasons. An ongoing debate in the platelet biology community is whether α-granules are heterogeneous or homogenous with regard to cargo. Defining the molecular underpinnings of α-granule biogenesis will help address this question. This line of inquiry will also inform efforts to load α-granules with exogenous proteins to target special cargos to thrombi, tumors, or wounds.9 Opportunities for antiplatelet therapy could arise from selective targeting of components of platelet granulogenesis pathways. Although unraveling the vesicle trafficking events responsible for α-granule formation is a daunting task and our current knowledge remains rudimentary, the approaches used by Bem et al point a way to a more mechanistic understanding of platelet granule formation.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal