In this issue of Blood, Copie-Bergman et al demonstrate that MYC rearrangements (MYC-Rs) with IG genes, but not with other partner genes, have a negative prognostic impact in patients with diffuse large B-cell lymphomas (DLBCLs) treated with immunochemotherapy.1
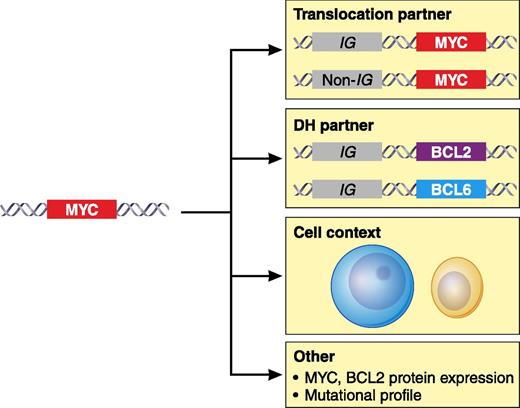
The biological effect of MYC translocation in DLBCL may be modulated by additional parameters that include the IG or non-IG partner gene involved in the translocation, the association with an additional translocation of the BCL2 or BCL6 genes in the so-called DH, the tumor cell context in which the alteration occurs such DLBCL, GCB or ABC, BCLu, and other factors such as the MYC or BCL2 protein levels. Additional studies are needed to explore other aspects such as the influence of somatic mutations in the tumor. Professional illustration by Patrick Lane, ScEYEnce Studios.
The biological effect of MYC translocation in DLBCL may be modulated by additional parameters that include the IG or non-IG partner gene involved in the translocation, the association with an additional translocation of the BCL2 or BCL6 genes in the so-called DH, the tumor cell context in which the alteration occurs such DLBCL, GCB or ABC, BCLu, and other factors such as the MYC or BCL2 protein levels. Additional studies are needed to explore other aspects such as the influence of somatic mutations in the tumor. Professional illustration by Patrick Lane, ScEYEnce Studios.
MYC is a powerful oncogene involved in the pathogenesis of aggressive lymphoid neoplasms usually activated by gene translocations.2 MYC translocations are considered the primary genetic event in Burkitt lymphoma (BL) but they also occur in 5% to 15% of DLBCLs, 30% to 50% of B-cell lymphomas (BCLs) unclassified with features intermediate between DLBCL and BL (BCLu), and a small proportion of DLBCLs transformed from small BCLs.2 MYC alterations commonly occur in the context of other oncogenic events that seem to cooperate in the transformation process. Interestingly, MYC translocations in DLBCL are frequently associated with BCL2 or, to a lesser extent, BCL6 translocations, in the so-called “double-hit” (DH) lymphomas (see figure).
MYC translocations in DLBCL have recently been reported to identify a subset of patients with an unfavorable prognosis. The failure of current immunochemotherapy protocols to control the disease in these patients has motivated the development of new therapies that may overcome the adverse clinical impact of this genetic alteration.3 However, one of the major challenges in advancing this perspective is understanding the complex biological mechanisms underlying the relationship between MYC alterations and the behavior of the tumors. Although most studies highlight the adverse impact of MYC translocations in DLBCL, some reports have provided conflicting results questioning whether MYC translocation as a single hit (SH) or its frequent association with BCL2 or BCL6 (DH) alterations is responsible for the aggressive behavior.4 Some studies have suggested that the BCL6 translocation in DH lymphomas may not have the same meaning as BCL2 alterations.5 On the other hand, some patients carrying MYC translocations or even DH alterations survive for long periods of time, raising the possibility that additional factors may modulate the adverse effect of MYC activation.4
The analysis of the literature is difficult. Many studies are retrospective and analyze patients treated with and without immunochemotherapy or combine primary, transformed, and relapsed DLBCL with MYC translocations. The distinctions between DLBCL and BCLu or the molecular subtypes of DLBCL, germinal center B-cell–like (GCB) or activated B-cell type (ABC), are not always considered. In addition, preliminary studies suggest that some biological aspects such as the partner gene in the MYC translocation or the levels of MYC or BCL2 protein expression may also influence tumor behavior.2,4,6
The interplay of so many variables and the relative low frequency of aggressive lymphomas with MYC alterations make the development of appropriate studies challenging. However, this is exactly what Copie-Bergman and colleagues do in their study.1 The authors concentrated (in the evaluation of the MYC translocation partner, IG vs non-IG gene) on the outcome of 574 patients with DLBCL treated with immunochemotherapy in the context of clinical trials. They started using a MYC break-apart fluorescence in situ hybridization probe to detect any MYC translocation followed by IGH, IGK, and IGL fusion probes to confirm whether the partner was an IG or non-IG gene. MYC analysis was combined with the investigation of BCL2 and BCL6 translocations. MYC-Rs were found in 9% of the cases with a similar distribution of IG (48%) or non-IG (52%) as partner genes. Interestingly, only the rearrangement with IG had a negative effect on the outcome of the patients, and this impact was seen in cases with isolated MYC translocation and also in DH tumors. Concordant with previous messenger RNA studies,7 the authors found significantly higher MYC protein expression in MYC-IG than in MYC-non-IG translocated cases, suggesting that MYC levels and its transcriptional regulator partner may be relevant in the behavior of the tumor.
The study further clarifies other controversial issues. The prognostic impact of MYC-SH and MYC-DH was only observed in DLBCL with a GCB phenotype, suggesting that MYC activation may be more relevant in this subset of DLBCL. Intriguingly, MYC-SH translocations but not MYC-DH had an independent prognostic value from the International Prognostic Index or cell-of-origin classification. However, when MYC/BCL6-DH cases were excluded, then MYC/BCL2-DH also had an independent poor prognostic impact. The study included only 7 cases with MYC/BCL6-DH but, interestingly, 6 of them were non-GCB and had a tendency to better prognosis than cases with MYC/BCL2-DH, which occurs almost exclusively in GCB tumors. Previous reports on the prognostic value of MYC/BCL6-DH have been controversial,5,8 but the findings in the current study suggest that MYC/BCL6 may have a different biological meaning than MYC/BCL2 in DH tumors.
One intriguing aspect in the Copie-Bergman et al study is the better-than-expected outcome of patients with MYC alterations, independent of the translocated partner or subtype of DH lesions, compared with the poor outcome reported in the majority of previous publications.1-4 As the authors recognize, the selection of patients from clinical trials may represent a bias that excludes patients with a poor performance status from being eligible for the trials. In fact, virtually all cases in their study had DLBCL morphology and, upon review, only 4 cases qualified for BCLu. This is a subset of cases with frequent DH lesions and very aggressive behavior. This observation would also support previous studies suggesting that the morphology of the tumor (DLBCL vs BCLu) may be an additional element that also matters in the evaluation of the effect of MYC alterations.4,9
In summary, Copie-Bergman et al and other studies are starting to clarify the different factors, such as gene translocation partners, protein expression, or tumor subtypes, that may modulate the biological and clinical effect of MYC translocations in aggressive lymphomas. Understanding their interplay is essential to fulfilling the goal of providing the most appropriate therapy. However, the role of other factors, such as the profile of somatic mutations,10 will also most probably be needed to complete this complex landscape.
Conflict-of-interest disclosure: The author declares no competing financial interests.