Key Points
Enterocytes of patients with aGVHD undergo dramatic telomere shortening (∼200 bp/wk).
Telomere loss with subsequent replicative exhaustion might provide a mechanism for refractory gut GVHD.
Abstract
Acute intestinal graft-versus-host disease (aGVHD) refractory to immunosuppressive treatment is a serious complication after allogenic hematopoietic stem cell transplantation (HSCT). The underlying mechanisms of refractory aGVHD of the gut are not fully understood. Although telomere length (TL) reflects the replicative history of a cell, critically short telomeres have been associated with replicative exhaustion and tissue failure. In this study, we demonstrate that enterocytes of patients with refractory intestinal aGVHD show significantly increased proliferation, which translates into significant and critical telomere attrition following HSCT as compared with unaffected patients undergoing HSCT. Calculated telomere loss in aGVHD patients is 190 bp/wk, thereby massively exceeding physiological steady-state TL shortening rates such as in lymphocytes (∼50 bp/y). Our data support the hypothesis that increased compensatory proliferation following continued tissue damage can result in massive telomere loss in enterocytes of aGVHD patients. The present study introduces aGVHD-triggered increased cellular turnover and telomere loss with subsequent replicative exhaustion as a mechanism for refractory gut GVHD that is compatible with the long-term clinical aspect of the disease and provides a basis for stem cell protective therapies in the treatment of aGVHD.
Introduction
Telomeres consist of repetitive DNA sequences at the end of the chromosomes and protect the DNA from degradation and fusions.1 Because of the end-replication problem, telomeres shorten with every cell division, and cells enter cellular senescence when telomeres become critically short. Thus, telomere length (TL) both reflects and limits the replicative capacity of cells.1,2 As a consequence, replicative senescence can be observed in tissues with impaired telomere maintenance providing a pathophysiological mechanism for organ failure.1,2
Acute intestinal graft-versus-host disease (aGVHD) is one of the major complications after allogeneic hematopoietic stem cell transplantation (HSCT).3 The underlying pathophysiology of aGVHD is poorly understood,4 but T-cell–triggered apoptosis of the crypt cells contributes to disease severity.5 Despite improvements of therapy, treatment-related mortality is high, especially in patients with refractory aGVHD.3 One possible explanation for treatment refractoriness could be the continued immune-mediated depletion of the intestinal (stem cell) compartment inducing increased compensatory proliferation and subsequent telomere attrition in the remaining intestinal crypt (stem cells) pool eventually resulting in replicative exhaustion.
In our study, we set out to analyze proliferation and TL in patients with and without aGVHD. For TL analysis, we used confocal microscopy in combination with quantitative fluorescent in situ hybridization (Q-FISH), a technique allowing the accurate measurement of single telomeres in individual cells along with precise identification of tissue morphology in paraffin-embedded samples.6 We demonstrate that telomere-mediated replicative exhaustion can contribute to treatment failure suggesting a role for the stem cell (and telomere) protective treatment strategies in addition to immunosuppressive therapy.
Study design
Retrospective analysis was carried out in formalin-fixed paraffin-embedded intestinal biopsies (archive material) obtained from colonoscopies or sigmoidoscopies of 25 patients enrolled in the allogeneic HSCT program of the University Hospital Regensburg. Patient informed consent was approved by the local institutional Ethics Committee, in accordance with the Declaration of Helsinki. Diagnosis and grading of aGVHD was carried out based on consensus criteria.3 First biopsy was acquired in median within 25 days (range 14-127 days) after HSCT in 15 patients with aGVHD and within 25 days (range 15-715 days) in 10 control patients without aGVHD. Second biopsy was acquired in median within 130 days (range 76-301 days) after HSCT in 15 patients with aGVHD and within 195 days (range 120-280 days) in 3 control patients without aGVHD. Biopsies of GVHD patients originated from sites adjacent to ulcerations and denuded mucosa. Biopsies of control patients were taken during a routine sigmoidoscopy after engraftment in the absence of intestinal GVHD signs. The follow-up samples were taken under persistence of aGVHD symptoms. Detailed clinical characteristics of the patients are shown in supplemental Table 1 (see the Blood Web site).
TL was assessed by Q-FISH, as previously described.7-9 All experiments were carried out single blinded, and images were captured under identical experimental conditions. Images were acquired with a confocal laser scanning microscope (LSM 710; Zeiss, Germany) running the Zen 2009 (Zeiss) software. A 63× 1.4 NA oil immersion objective was used for image acquisition. Image processing was performed using Definiens Developer software (XD 64, Cell Porta). GraphPad Prism 5.0 (GraphPad) was used for data analysis. Further details are provided in supplemental Methods. TL is given as mean value of all detected telomere spots in the analyzed enterocytes. TL in kilobase pairs was estimated by linear regression of TL measurements on 5 paraffin-embedded control cell specimens with defined TL. Results are presented as mean ± standard error of the mean, statistical significance is set at P < .05.
Immunostainings of the proliferation marker Ki-67 (MIB-1 antibody) were available for 21 patients according to the streptoperoxidase-based standard protocols established at the Institute of Pathology of the University Hospital Regensburg.
Results and discussion
In order to investigate the replicative history of enterocytes in the colonic crypts of patients with aGVHD, we first analyzed whether aGVHD leads to increased proliferation. For this purpose, we used the semiquantitative scoring system for the proliferation marker Ki-67. MIB-1 scoring at the time of the first biopsy was significantly higher in aGVHD (1.87 ± 0.19, n = 12) than in control enterocytes (1.22 ± 0.20, n = 9; P = .04; Figure 1A-B). Interestingly, MIB-1 scoring of the follow-up biopsies in aGVHD patients was lower yet not statistically different (1.46 ± 0.18, n = 12, P = .08) compared with the initial scoring value (supplemental Figure 1A).
Increased cell proliferation in patients with aGVHD. (A) Representative MIB-1 immunohistochemical staining of the initial gut biopsies of a control (left) and an aGVHD patient (right). Shown magnification is ×20. (B) Immunohistochemical scoring of the MIB-1 staining shows a significant increase of proliferating enterocytes in aGVHD patients compared with controls.
Increased cell proliferation in patients with aGVHD. (A) Representative MIB-1 immunohistochemical staining of the initial gut biopsies of a control (left) and an aGVHD patient (right). Shown magnification is ×20. (B) Immunohistochemical scoring of the MIB-1 staining shows a significant increase of proliferating enterocytes in aGVHD patients compared with controls.
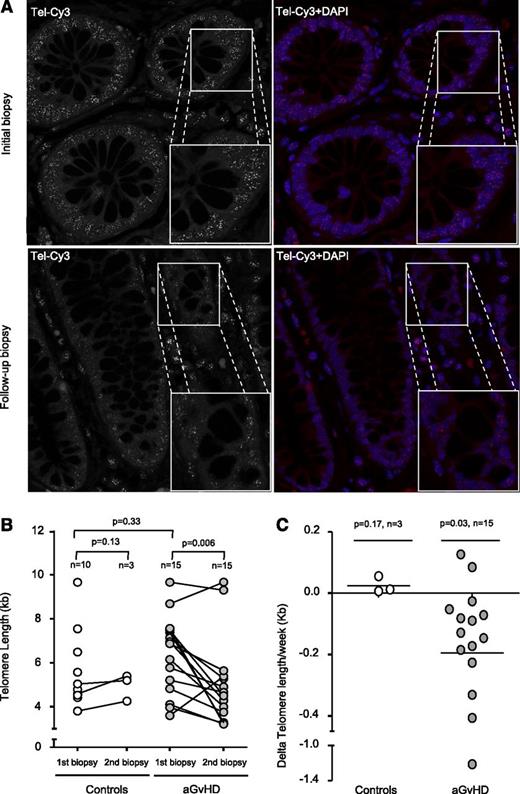
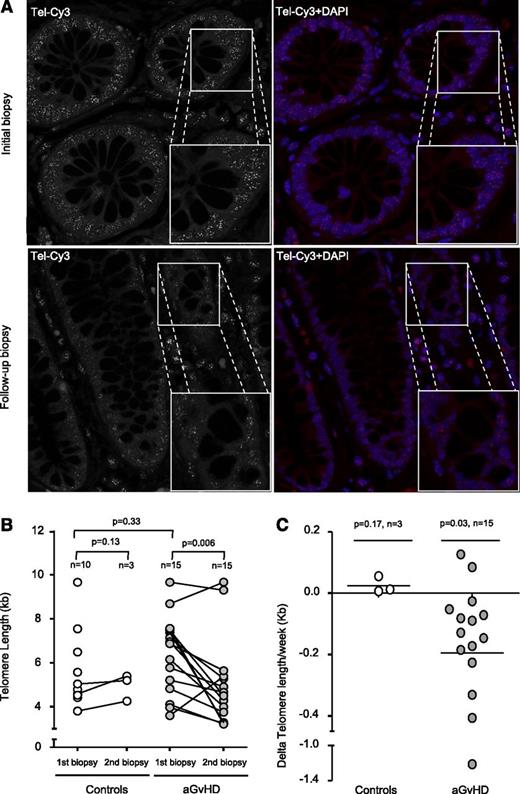
Next, we investigated the effects of the increased proliferation on TL in the intestinal stem cell compartment. Putative stem cells of the intestine can be found between the Paneth cells located at the bottom and the transiently amplifying cells located at the top of the Lieberkühn crypts.10 Recent studies demonstrated that TL of these cells strongly correlates with the respective putative stem cell intestinal pool.11,12 In our study, cells of the colonic crypts localized within the transient amplifying cell region of the crypts and differentiated enterocytes were analyzed (supplemental Figure 2A). As TL declines with age, we first analyzed age distribution of control and aGVHD patients. We found no significant difference between control (46.5 ± 3.3 years) and aGVHD patients (48.5 ± 2.6 years, P = .97), thus providing the basis for a direct comparison of TL between patients of both groups (supplemental Figure 1B). TL of control patients (5.6 ± 0.6 kb, n = 10) and the first biopsy of aGVHD did not significantly differ (6.3 ± 0.5 kb, n = 15, P = .33; Figure 2B). However, when we analyzed patients with refractory aGVHD, TL of follow-up biopsies was significantly shorter (4.95 ± 0.5 kb, n = 15, P = .006) compared with the respective initial TL (Figure 2A-B). Because of the obvious difficulties of obtaining follow-up biopsy material from control patients after the first biopsy, only 3 out of the 10 control patients underwent follow-up biopsies that were available to us, showing no significant TL shortening (P = .13). Whereas aGVHD patients show a dramatic telomere loss of −0.19 ± 0.08 kb/wk (n = 15), controls patients (n = 3) maintained TL at a constant level during the follow-up period post-HSCT (0.02 ± 0.01 kb/wk; Figure 2C). We observed no significant correlation between clinical data and respective telomere loss of aGVHD patients most likely because of the small sample size.
Telomere shortening in aGVHD patients. (A) Representative confocal microscopy images of the 2 gut biopsies from an aGVHD patient analyzed by Q-FISH. The upper panels represent the initial biopsy, and lower panels the follow-up biopsy. Telomeres were stained with Tel-Cy3 peptide nucleic acid probe, and chromatin was stained with 4,6 diamidino-2-phenylindole (DAPI). Black-and-white images of the telomere signal are shown to improve comparability. Micrograph magnification is ×100. (B) Longitudinal analysis shows that TL is significantly reduced in aGVHD patients but not in controls. One circle corresponds to 1 biopsy. One connecting line corresponds to 1 individual patient. (C) Telomere shortening is shown for the enterocytes of aGVHD patients with a mean telomere loss rate of −0.19 per week compared with enterocytes of the control.
Telomere shortening in aGVHD patients. (A) Representative confocal microscopy images of the 2 gut biopsies from an aGVHD patient analyzed by Q-FISH. The upper panels represent the initial biopsy, and lower panels the follow-up biopsy. Telomeres were stained with Tel-Cy3 peptide nucleic acid probe, and chromatin was stained with 4,6 diamidino-2-phenylindole (DAPI). Black-and-white images of the telomere signal are shown to improve comparability. Micrograph magnification is ×100. (B) Longitudinal analysis shows that TL is significantly reduced in aGVHD patients but not in controls. One circle corresponds to 1 biopsy. One connecting line corresponds to 1 individual patient. (C) Telomere shortening is shown for the enterocytes of aGVHD patients with a mean telomere loss rate of −0.19 per week compared with enterocytes of the control.
Our study analyzed for the first time telomere dynamics in the intestine of patients with aGVHD. We observed increased proliferation and consecutive massive telomere shortening in patients with aGVHD. Interestingly, the observed shortening of ∼200 bp/wk exceeds impressively the previously reported loss of telomeres in other tissues2 (eg, in human blood lymphocytes with mean shortening of ∼40-50 bp/y).
Based on our data, we propose that intestinal stem cells initiate compensatory hyperproliferation in response to the destruction of the intestinal mucosa (supplemental Figure 2A). The aGVHD-triggered hyperproliferation exceeded the capacity of the stem cell compartment to maintain the TL leading to telomere-mediated replicative exhaustion.
Our model is supported by a previous study reporting a mouse model with a conditional depletion of the stem cell compartment of the intestine leading to intestinal failure caused by selective increased cell turnover, consequent telomere shortening, and cellular senescence.13 Similar findings can be observed in patients with dyskeratosis congenita, a disease characterized by altered telomere maintenance. Dyskeratosis congenita patients show signs of enteropathy and apoptosis because of premature critical shortening of telomeres.14 Further supporting our data, patients with active colitis ulcerosa show premature telomere shortening in comparison with asymptomatic patients, highlighting the role of inflammation as a cause of replication-dependent telomere shortening.15
We postulate that replicative exhaustion and subsequent cellular senescence of the intestinal stem cell compartment can at least partially explain clinical refractoriness of aGVHD despite sufficient immunosuppressive treatment. Further studies focusing on the analysis of nonrefractory aGVHD (milder disease) patient material, which is difficult to obtain because of obvious reasons, are warranted to reveal whether the degree of TL can be reversed in this patient population. The investigation of eventual alterations on the biology of the intestinal epithelium (ulcerated mucosa vs mucosa remote from lesions) would additionally clarify the clinical impact of replicative exhaustion in aGVHD.
Based on our findings, we speculate that the treatment strategy of aGVHD should be reviewed and modified when justifiable to include either telomerase-activating therapies (eg, oxymetholon used for aplastic anemia patients16,17 ) or stem cell protective treatment approaches (eg, IL-2218 ) in order to prevent the replicative exhaustion of the intestinal stem cell compartment before achievement of sufficient control of the inflammatory process.
In summary, our work provides new perspectives for the treatment of aGVHD centered on stem cell–based therapies for deceleration of telomere shortening in intestinal colon crypts.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Immunohistochemistry and Confocal Microscopy Unit, a core facility of the Interdisciplinary Center for Clinical Research Aachen within the Faculty of Medicine at Rheinisch-Westfälische Technische Hochschule Aachen University, and Julian Kaufmann for technical assistance.
F.B. was supported by the START program of the University Hospital Aachen.
Authorship
Contribution: S.H. performed experiments, collected data, wrote the manuscript, and analyzed the data; M.S.V.F. analyzed and interpreted the data and wrote the manuscript; E. Huber performed and analyzed the histopathology; D.H., E. Holler, and K.S. contributed to clinical data and biopsy collection, as well as writing of the manuscript; D.F., F.G., and G.M.-N. performed experiments and analyzed the data; P.Z., E.J., M.A.B., and T.H.B. analyzed and interpreted the data, wrote the manuscript, and provided financial support; F.B. conceived and planned the study design, performed experiments, interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Fabian Beier, Department of Hematology, Oncology and Stem Cell Transplantation, University Hospital Aachen, Pauwelsstrasse 30, 52074 Aachen, Germany; e-mail: fbeier@ukaachen.de.
References
Author notes
S.H. and M.S.V.F. contributed equally to this study.