Abstract
Introduction: Hemophilia A is an X-linked bleeding disorder characterized by a deficiency or absence of blood coagulation protein factor VIII (fVIII). Inhibitor development is the most common complication of fVIII treatment occurring in up to 30% of individuals with severe hemophilia A. Although the C2 and A2 domains are considered the predominant immunogenic domains of fVIII, prior work in our lab using a hemophilia A mice model immunized with fVIII showed that ~10% of the 506 hybridomas isolated produced antibodies to the C1 domain. Further studies have shown that the C1 domain participates in binding to activated platelets, phospholipid membranes, and von Willebrand factor (VWF) as well as fVIII uptake by dendritic cells. Two recent studies postulated that VWF primarily interacts with fVIII at the C1 domain using negative-stain electron microscopy. However the pathogenicity of anti-C1 antibodies have yet to be fully explored.
Methods: E16 knockout hemophilia A mice were immunized with B-domain deleted fVIII. Spleens from the mice were harvested and fused with NS-1 myeloma cells to produce hybridomas using standard Köhler-Milstein technology. Hybridomas producing antibodies to the C1 domain were isolated and purified for monoclonal antibodies (MAbs). The effect of these anti-C1 MAbs on fVIII function and bleeding phenotype in mice were analyzed.
Results: We have produced 8 anti-C1 MAbs from 5 different spleens. Two non-overlapping B-cell epitopes were identified, group A and B, using a competition sandwich ELISA. The 7 group A MAbs recognize an overlapping B-cell epitope despite 5 different spleens of origin, providing evidence for an immunodominant B-cell epitope. The group A MAbs weakly inhibit fVIII with inhibitor titers of < 180 BU/mg IgG by Bethesda assay. In contrast anti-A2 MAb 4A4 has an inhibitor titer of 40,000 BU/mg IgG. Five of these 7 MAbs inhibit fVIII binding to VWF and 1 MAb inhibits fVIII binding to phospholipids by competition ELISA. MAb B136, a group B MAb, binds to an epitope distinct from the group A MAbs and has an inhibitor titer of 700 BU/mg IgG. An additional human-derived anti-C1 MAb KM33 competes with group A and B MAbs and has an inhibitor titer of 97 BU/mg IgG. MAbs B136 and KM33 potently inhibit fVIII binding to VWF and phospholipids. All of the anti-C1 MAbs have a strong binding affinity to fVIII with dissociation constants (KD) of 0.1 - 10 nM determined by surface plasmon resonance (SPR) spectroscopy despite relatively low titers of inhibition (Table 1). MAbs 2A9, F156, I84, M6143, and B136 also inhibit peak thrombin generated and endogenous thrombin potential in the thrombin generation assay and fVIIIa incorporation into the intrinsic Xase assay in a similar fashion as the one-stage clotting assay.
| MAb . | Group . | Spleen Origin . | Inhibitor titer (BU/mg IgG) . | VWF Binding IC50 (µg/ml) . | Phospholipid Binding IC50 (µg/ml) . | KD (nM) . | Median Blood Loss/Body Weight (mg/g) . |
|---|---|---|---|---|---|---|---|
| 2A9 | A | 1 | 23 | 1.1 | 0.9 | 0.9 | 29.8 |
| B153 | A | 2 | 3 | >10 | >10 | 10 | - |
| F156 | A | 3 | 7 | >10 | >10 | 2.9 | 1.7 |
| I41 | A | 4 | 15 | 2.7 | >10 | 1.9 | - |
| I84 | A | 4 | Indeterminate | 3.3 | >10 | 1.9 | - |
| I88 | A | 4 | 3 | 1.7 | >10 | 1.0 | - |
| M6143 | A | 5 | 180 | 0.6 | >10 | 0.2 | 41 |
| B136 | B | 2 | 700 | 0.4 | 0.04 | 0.1 | 43.9 |
| KM33 | AB | N/A | 97 | 0.03 | - | 0.1 | 45.9 |
| MAb . | Group . | Spleen Origin . | Inhibitor titer (BU/mg IgG) . | VWF Binding IC50 (µg/ml) . | Phospholipid Binding IC50 (µg/ml) . | KD (nM) . | Median Blood Loss/Body Weight (mg/g) . |
|---|---|---|---|---|---|---|---|
| 2A9 | A | 1 | 23 | 1.1 | 0.9 | 0.9 | 29.8 |
| B153 | A | 2 | 3 | >10 | >10 | 10 | - |
| F156 | A | 3 | 7 | >10 | >10 | 2.9 | 1.7 |
| I41 | A | 4 | 15 | 2.7 | >10 | 1.9 | - |
| I84 | A | 4 | Indeterminate | 3.3 | >10 | 1.9 | - |
| I88 | A | 4 | 3 | 1.7 | >10 | 1.0 | - |
| M6143 | A | 5 | 180 | 0.6 | >10 | 0.2 | 41 |
| B136 | B | 2 | 700 | 0.4 | 0.04 | 0.1 | 43.9 |
| KM33 | AB | N/A | 97 | 0.03 | - | 0.1 | 45.9 |
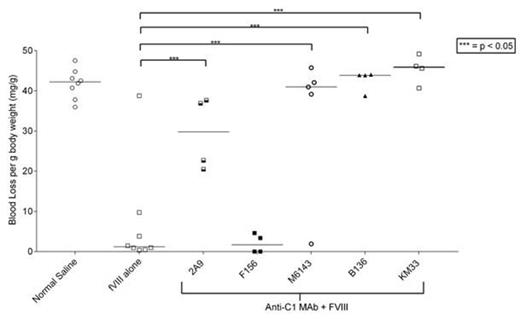
The pathogenicity of anti-C1 MAbs were assessed in a tail snip bleeding model. Hemophilia A mice received injections of anti-C1 MAb at saturating levels of 0.5 mg/kg (~65 nM) followed by 180 U/kg (~2.5 nM) fVIII and tail transection 2 hours later. Mice that received MAbs 2A9 (group A), M6143 (group A), B136 (group B), and KM33 (group AB) induced bleeding with median blood loss per mouse body weight of 29.8, 41, 43.9, and 45.9 mg/g respectively compared to 1.2 mg/g in mice who received fVIII alone (p = 0.049, 0.011, 0.008, and 0.004 respectively, Mann-Whitney). MAb F156 did not induce bleeding. MAbs F156, 2A9, M6143, B136, and KM33 produced differing inhibitor titers of 0.01, 0.3, 2.3, 8.8, and 1.2 BU/ml respectively in this system. Despite low inhibitor titers of 0.3, 2.3, and 1.2 BU/ml and a high fVIII dose of 180 U/kg, MAbs 2A9, M6143, and KM33 induced bleeding in mice. Previously we have shown that group A MAbs with epitopes that overlap 2A9 were found in 42% of patients with hemophilia A and inhibitors.
Conclusion: Although anti-C1 MAbs show weak inhibition in in-vitro assays and low inhibitor titers, they bind tightly to fVIII and induce bleeding in a tail snip model. These pathogenic low titer inhibitors are found in patients with hemophilia A and inhibitors.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.