Abstract
The ECOG-ACRIN Cancer Research Group Led Trial, E2902, was a randomized phase III North American Leukemia Intergroup trial of the Farnesyl Transferase Inhibitor , R115777(Tipifarnib), as maintenance therapy for acute myeloid leukemia (AML). Patients (pts) with AML in remission after requiring salvage therapy (primary induction failure or second or subsequent remission) or over the age of 60 in first remission were eligible. Pts were eligible if they were in a complete remission (CR) or complete remission without platelet recovery (CRp) as confirmed by bone marrow examination within 2 weeks prior to randomization. Consolidation or post remission therapy prior to enrollment was allowed but not required. Pts who had received an allogeneic transplant in this remission were ineligible. Adequate blood counts (absolute neutrophil count >= 1000/mm3 and platelet count >=50,000/mm3), and normal renal, and hepatic function were required within 2 weeks of randomization.
Pts were randomized to either receive tipifarnib twice daily (BID) or to observation. Pts were stratified by remission ( CR1 vs > CR1) and whether or not they received consolidation therapy in the current remission. The main objective of this study was to compare disease free survival(DFS) in the two groups. Between August 2004 and December 2009, 144 pts were accrued to the study. The median age of the pts was 69 (range 28-86). Seventy-three pts (51%) were male and 135 pts (94%) were white. The majority of pts enrolled on the study (70%) were in first CR, and had post remission chemotherapy (56%) prior to randomization. Seventy-three pts were enrolled onto the treatment arm (Arm A) and 71 pts were enrolled onto the observation arm (Arm B). When the study was initially opened, pts on Arm A were treated with tipifarnib at a dose of 400 mg BID with dose reductions and interruptions defined per protocol for toxicity. After the first planned interim analysis, based on toxicity data, the ECOG Data Monitoring Committee recommended that the starting dose be decreased to 300 mg BID. While non-hematologic toxicity was acceptable, significant hematologic toxicity was seen in pts treated at an initial dose of either 400 mg or 300 mg BID. Of note, hematologic toxicity was also seen on the observation arm although less frequently than with both treatment doses.
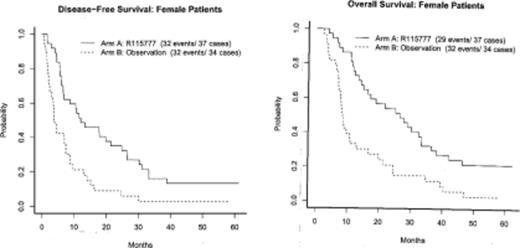
The primary endpoint of the study was analysis of DFS on an intent to treat basis. The median DFS for arms A and B are 8.87 months and 5.26 months respectively ( p=0.058, HR 0.760). When restricted to eligible patients only(n=134 ), the DFS for arms A and B are 10.81 vs 5.26 months, respectively ( p=0.019, HR 0.690). An unplanned subgroup analysis was performed by gender. The median DFS for male pts (n=73) is 5.36 vs 5.91 months for arms A and B respectively (p=0.868, HR 1.320) and 12.09 vs 3.91 months for female pts (n=71)(p=0.0002, HR=0.408) In a multivariate Cox proportional hazards model to adjust for baseline clinical variables, there is a significant interaction between treatment and gender. (Table 1) Similar results were noted for OS (Figure 1).
ECOG ACRIN Trial E2902 evaluated the potential role of Tipifarnib ( R115777) in patients with AML in remission with a high risk of relapse. While the primary endpoint for improved DFS was not reached when evaluating all randomized pts, when restricted to eligible patients only, there is a statistically significant improvement in DFS seen. Moreover, an unplanned subgroup analysis by gender demonstrates an improvement in DFS and OS for females who were enrolled on the study which persists on multivariate analysis. Possible explanations include the use of flat dosing rather than BSA based dosing on this study. However futher evaluation of patient and disease characteristics, dosing and toxicity profiles of responders and nonresponders is needed. Given what appears to be a potentially clinically relevant benefit, further evaluation of this agent is warranted.
Multivariate Cox Proportional Hazards Model for DFS: All Randomized Patients
| . | Hazard Ratio (95% CI) . | P value . |
|---|---|---|
| Arm B vs A | 2.559(1.547,4.236) | 0.00025 |
| Male vs Female | 2.005(1.228,3.274) | 0.00544 |
| CR1 vs >CR1 | 0.447 (0.305,0.656) | 0.00004 |
| . | Hazard Ratio (95% CI) . | P value . |
|---|---|---|
| Arm B vs A | 2.559(1.547,4.236) | 0.00025 |
| Male vs Female | 2.005(1.228,3.274) | 0.00544 |
| CR1 vs >CR1 | 0.447 (0.305,0.656) | 0.00004 |
Rowe:BioSight Ltd.: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy; BioLineRx Ltd.: Consultancy. Larson:Astellas: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.