Abstract
Introduction: Syncytial variant of nodular sclerosis HL (SV) is a well-described distinct pathologic entity characterized by prominent aggregates of Hodgkin/Reed- Sternberg cells in nodules separated by fibrous collagen bands. Despite its well-known morphologic description, little is known regarding the clinical behavior of the SV. Previous reports suggest that patients with SV often presented with B symptoms and advanced stage disease, however, large series defining the clinical outcome have not been reported so far. We systematically studied the clinical features and outcome of patients with SV and further compared them with patients with typical nodular sclerosis HL (t-NS).
Patients and Methods: 167 adult patients (pts.) with Nodular Sclerosis HL were included in our analysis following institutional IRB approval. Differences between the two groups (SV vs. t-NS) were analyzed using Chi- square and t -Student tests. Statistical significance was set at P <0.05. Kaplan Meier method was used to calculate the Progression-free survival (PFS) and overall survival (OS). Log-rank test was used to determine the differences in survival. Statistical analysis was performed using SPSS.22 software.
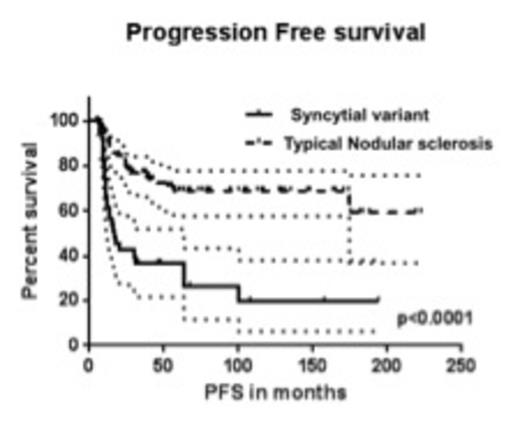
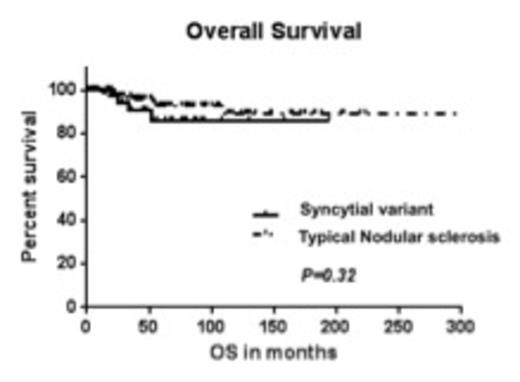
Results: Of the 167 patients, 43 were confirmed as SV based on morphology and immunophenotype. The median age at diagnosis was 31 yrs. (range: 18-75 yrs.) and 85 patients were male (51%). At diagnosis, 100% patients had an ECOG status of 0-1; 39 % had advanced stage disease (stage III and IV); and 48% had B symptoms. 23 % patients presented with bulky disease defined as a mass > 10 cm or a mediastinal mass >1/3 of thorax at T5-T6. 90% patients received ABVD as their initial treatment. The remaining were treated with Stanford V, MOPP or on a clinical trial. 37 % patients received radiation therapy. Furthermore, 63% patients with SV and relapsed disease were treated with high dose therapy followed by stem cell transplant (ASCT). In the SV vs. t-NS comparison, no statistically significant differences were observed between the two groups with regards to age, gender, stage at presentation, B symptoms, bulky disease or favorable features. The rate of complete response (CR) in the SV group was 74% vs. 87% in the t-NS group (P=0.05). Moreover, at a median follow up of 49 months, the median progression free survival (PFS) was inferior in the SV group (17.02 months) compared with the t-NS group (not reached) (P<0.0001; HR = 3.695; 95% CI=3.0-11.07). The median overall survival (OS) was not reached in both groups and was not statistically different [Fig.1&2, P=0.32]. Significant differences in PFS and OS were observed based on stage of disease (early vs. advanced) and achievement of complete response at end of treatment by univariate analysis. In the corresponding multivariate analysis, achievement of CR following completion of treatment was the only independent predictor for OS.
Discussion: In summary, our results suggest that SV was associated with a lower rate of complete response to standard ABVD chemotherapy +/- radiation and inferior PFS. Despite the high rate of relapse associated with SV, these patients can be salvaged with standard salvage regimens, ASCT or newer immunomodulatory agents and therefore not compromising OS. Our report suggests that patients with SV should be considered for novel combination immuno-chemotherapies to improve the rate of complete remission and subsequently avoid the need for ASCT.
Reddy:PCYC: Consultancy; ImmunoGen: Consultancy; Gilead: Other: Speaker; Seattle Genetics: Consultancy; Celgene: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract