Abstract
[Introduction] Oxidative stress caused by the increased production of reactive oxygen species (ROS) or decreased efficacy of the antioxidant system is implicated in the pathogenesis of various disease entities, such as atherosclerosis, cardiovascular disease, renal failure, malignant tumors, and autoimmune diseases. Chemotherapy and radiation therapy are associated with increased formation of ROS. Conditioning regimens preceding hematopoietic stem cell transplantation (HSCT) usually consist of high-dose chemotherapy and/or total body irradiation (TBI). A limited number of studies demonstrated that the conditioning therapy given to HSCT patients creates a high oxidative stress and decreases the antioxidant defense system. The objective of this study was to look for further evidence of oxidative stress status in HSCT patients.
[Methods] In this study, urine samples were collected from 32 HSCT patients before and after conditioning therapy and from 15 healthy controls. The patients included 19 male and 13 female with a median age of 58 years (27-68 years). Twenty patients received allogeneic HSCT (9 uBMT, 1 rPBSCT and 10 CBT) and 12 patients received autologous PBSCT. The conditioning regimens for allo-HSCT included TBI12Gy+Ara-C+G-CSF (n=3), TBI12Gy+Ara-C+CY (n=1), TBI12Gy+VP-16+CY (n=1), Flu+L-PAM+TBI3Gy (n=6), Flu+BU+TBI3Gy (n=1), Flu+Ara-C+G-CSF+BU+TBI4Gy (n=3), Flu+L-PAM (n=2), and BU+CY (n=3). The diagnosis included AML (n=9), MDS (n=5), DLBCL (n=1), FL (n=2), T-ALL (n=1) and MM (n=2). Fifteen patients (75%) had high-risk disease at transplantation. Twelve multiple myeloma (MM) patients received L-PAM 200mg/m2 + autologous PBSCT. We measured urinary 8-hydroxydeoxyguanosine (8-OHdG) by competitive immunochromatography using a novel automatic oxidative stress analyzer, ICR-001 (Techno-Medica). 8-OHdG, which originates from damaged DNA repaired by non-specific endonucleases and specific glycosylates and is eliminated into urine, is widely used as a sensitive biomarker of oxidative stress.
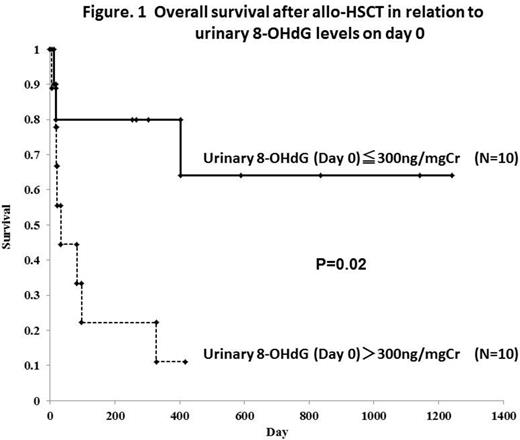
[Results] Urinary 8-OHdG significantly increased immediately after conditioning therapy. In 20 allo-HSCT patients, urinary 8-OHdG levels on day 0 were significantly higher than pre-conditioning levels and healthy controls (mean: 458.2 vs. 90.9 vs. 15.7 ng/mgCr). In 12 auto-HSCT patients, urinary 8-OHdG levels on day 0 were also higher than pre-conditioning levels (mean: 273.6 vs. 107.2 ng/mgCr). No significant correlation was found between urinary 8-OHdG levels and serum ferritin levels during pre- and post-transplant period. Allo-HSCT patients with high urinary 8-OHdG levels on day 0 (over 300ng/mg/Cr; N=10) had significantly shorter survival than those with low urinary 8-OHdG levels on day 0 (under 300ng/mg/Cr; N=10) (1-year OS, 11.1% vs. 80.0%, respectively; P=0.01) (Figure. 1). On the other hand, post-conditioning urinary 8-OHdG levels were not associated with outcome in auto-HSCT patients. In univariate analysis, a high 8-OHdG level on day 0, CBT, and RIC regimen were associated with poor OS in allo-HSCT patients.
[Conclusion] In the present study we demonstrated that conditioning therapy results in increased oxidative stress in HSCT patients. In particular, our data proved that high urinary 8-OHdG levels on day 0 were associated with poor prognosis in allo-HSCT patients. These results suggest that oxidative stress may have an important role in allo-HSCT and also may be a useful prognostic biomarker. Since our results are based on a small-sized analysis, further large prospective studies are warranted to verify this conclusion.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.