Abstract
Introduction: Approximately 10-15% of patients with DLBCL treated with R-CHOP chemotherapy have primary refractory disease at the completion of initial treatment. The standard treatment for patients with DLBCL in first relapse is rituximab and salvage chemotherapy. For those achieving chemosensitive remission, high-dose therapy and autologous stem cell transplant (ASCT) is the accepted standard of care. Data from three randomized studies in the rituximab era suggest that 40-50% of these patients are ultimately cured; however, the majority of patients in these studies did not have primary refractory disease. The outcomes and curability of primary refractory DLBCL patients in the rituximab era remain unknown.
Methods: We identified transplant-eligible patients from 2002 to 2014 with DLBCL that was refractory to initial rituximab and anthracycline containing regimens based on radiographic (16) and/or biopsy-proven (83) progression at the end of therapy. Patients with primary refractory disease were defined as either partial responders (partial response to initial therapy) or primary progressors (minimal or no response to initial therapy). The majority of patients were treated with three cycles of platinum containing regimens; 72% received R-ICE. High-dose therapy and ASCT were administered in 52% of cases. Response criteria were per IHP when pre- and post- salvage PET were available, otherwise by SPD per IWG (Cheson JCO 1999). The Kaplan-Meier method and Cox proportional hazards model were used to evaluate progression-free survival (PFS) and overall survival (OS).
Patient Characteristics: Ninety-nine patients were identified. Median age was 56 (range 22-73); 31% were older than 60. With respect to histology, 61% of patients had DLBCL-NOS, 17% had PMBL, 11% had transformed low-grade B cell lymphoma, 6% had T-cell rich B-cell lymphoma, 3% had grey zone lymphoma, and 2% had B-cell lymphoma unclassifiable. With respect to initial treatment response, 41% of patients were partial responders whereas 59% were primary progressors. An elevated LDH, Karnofsky Performance Status (KPS) <80 and Ann Arbor (AA) stage III-IV disease were seen at relapse in 67%, 17%, and 56% of patients, respectively. Bulky disease (>10 cm) and multiple (≥2) extranodal sites of disease were present in 18% and 35% of patients. An elevated second-line IPI (≥3) and aaIPI (≥3) were seen in 40% and 49% of patients, respectively.
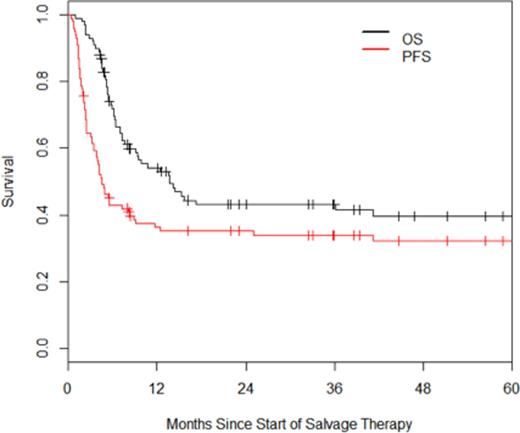
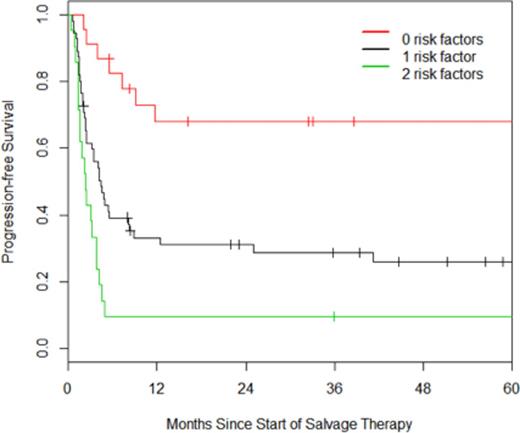
Outcomes: Median follow-up for survivors was 37 months. The overall response rate to salvage therapy was 56%, with 21% achieving a complete response and 35% achieving a partial response. As assessed by intention to treat (ITT), the estimated 3-year OS and PFS are 43% and 34% respectively (Figure 1). Within the ITT cohort, univariate analysis revealed that age ≥60, primary progressive disease, advanced AA stage, elevated LDH, KPS<80, multiple extranodal sites of disease, and an elevated second-line IPI were associated with inferior PFS, whereas PMBL was associated with superior PFS compared to DLBCL-NOS. Multivariate analysis revealed age ≥60 and an elevated LDH to be independently associated with PFS; patients with zero or two of these risk factors had hazard ratios of 0.32 and 2.05 for disease progression as compared to patients with one of these risk factors (Figure 2).
With respect to the 51 patients proceeding to ASCT, median follow-up from the date of SCT was 48 months. The estimated 3-year OS and PFS are 67% and 63% respectively. Within the transplanted cohort, univariate analysis revealed disease bulk >10 cm and an elevated IPI to be associated with inferior outcomes.
Conclusion: This is the largest reported series of patients with primary refractory DLBCL treated with curative intent in the rituximab era. Salvage therapy with consolidative ASCT achieves long-term remissions in about 40% of patients. ASCT achieves long-term remissions in over 60% of patients. Therefore, the primary barrier to curative therapy in this population is achieving a sufficient response to salvage therapy, and future studies should be aimed towards increasing the response rate in this population. Older patients and patients with an elevated LDH respond poorly to conventional salvage therapy and investigational approaches may be more appropriate in this setting.
OS and PFS of patients with primary refractory DLBCL.
PFS of patients based on the presence of elevated age (60+) and/or elevated LDH.
PFS of patients based on the presence of elevated age (60+) and/or elevated LDH.
Moskowitz:Celgene: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Honoraria, Research Funding; Merck: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees. Matasar:Genentech: Consultancy; Spectrum: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.