Abstract
KEAP1-NRF2 system is a major regulator of cellular redox balance and xenobiotic metabolism. NRF2 is an inducible transcription factor, and KEAP1 is its negative regulator. Under normal conditions, NRF2 is poly-ubiquitinated by KEAP1-CUL3 complex and is degraded through proteasome. KEAP1 is a stress sensor and is inactivated by oxidative stress and metabolites of xenobiotics, which results in relief of NRF2 from the KEAP1-mediated suppression and thereby induces cytoprotective genes. In addition to this cytoprotective function of KEAP1-NRF2 system, emerging evidence suggests that their roles extend to cell proliferation and differentiation.
Hematopoietic stem cells (HSCs) are maintained quiescent under normal conditions, and are stimulated to proliferate and differentiate in response to environmental alterations. However, the underlying mechanisms of driving HSCs from quiescence into proliferation are still elusive. Here, we investigated whether the KEAP1-NRF2 system contributes to the stress-responsive proliferation of HSCs. In this study, we analyzed long-term HSCs (LT-HSCs), defined as Lin- Sca-1+ c-Kit+ (LSK) CD48- CD150+ cells, which possess high bone marrow (BM) reconstitution capacity. Short-term HSCs (ST-HSCs) and multipotent progenitor (MPP) cells were also defined as LSK CD48- CD150- and LSK CD48+ CD150- cells, respectively.
First, we examined Keap1 conditional knockout mice, Keap1F/F::Mx1-Cre (Keap1 CKO1), and compared to control mice, Keap1F/+ or Keap1F/+::Mx1-Cre. In the Keap1 CKO1 mice, ST-HSCs and MPPs were increased, whereas LT-HSCs were not changed in comparison to control mice. However, Keap1-deficient LT-HSCs exhibited less engraftment and reconstitution in the competitive bone marrow transplantation (BMT) assay, in which donor-derived LT-HSCs were transplanted with BM competitor cells (Fig. 1). In particular, the Keap1-deficient LT-HSCs were almost completely lost after secondary BMT. Importantly, the attenuated reconstitution capacity of LT-HSCs in the absence of Keap1 was clearly recovered by the additional Nrf2 deletion (Fig. 1), which indicates that constitutive activation of NRF2 causes dysfunction of LT-HSCs.
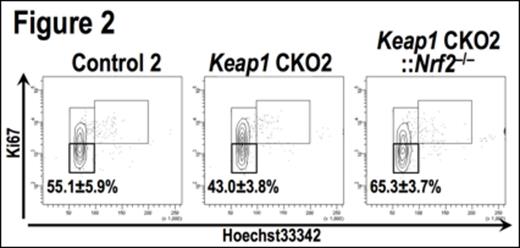
To elucidate the cause of the functional impairment in Keap1-deficient LT-HSCs, we examined homing potentials and apoptosis. However, there were no significant differences in either of the parameters. Next, we evaluated cell cycle status of LT-HSCs and found that Keap1-deficient LT-HSCs contained less quiescent cells, compared to control cells (G0 phase; Control = 63.6 ± 3.5%, Keap1 CKO1 = 55.2 ± 4.0%). This implies that Nrf2 activation enhances cell cycle entry of quiescent HSCs, which attenuates the stem cell activity. All these results observed in Keap1 CKO mice were reproduced in another Keap1 conditional knockout mice, Keap1F/F::Vav1-Cre (Keap1 CKO2). Of note, the reduction of quiescent LT-HSCs was nicely recovered by the additional Nrf2 deletion under the Keap1 CKO2 background (Fig. 2, G0 phase; Control2 = 55.1 ± 5.9%, Keap1 CKO2 = 43.0 ± 3.8%, Keap1 CKO2::Nrf2-/- = 65.3 ± 3.7%). These results show that NRF2 activation drives quiescent HSCs into cell cycling.
Since NRF2 exerts its activity in response to exogenous stimuli, we assessed whether the NRF2-mediated proliferation of LT-HSCs are induced by transient treatment with an NRF2 inducer, CDDO-Im. CDDO-Im treatment promoted cell cycling of LT-HSCs, which was ablated by Nrf2 deficiency, suggesting that NRF2 activation, even transiently, leads to cell cycle entry of quiescent HSCs. Finally, in order to elucidate contribution of NRF2 to LT-HSCs under steady-state conditions, we assessed Nrf2-deficient (Nrf2-/-) mice. No differences were observed in the BM of Nrf2-/- mice, but after the competitive BMT of LT-HSCs, Nrf2-deficient LT-HSCs exhibited less contribution to the BM reconstitution in the recipients. However, it is noteworthy that the rate of engrafted donor cells tends to be higher in the recipients transplanted with Nrf2-deficient LT-HSCs than in the recipients transplanted with wild-type cells after the secondary BMT, implying that NRF2, at least in part, contributes to cell cycle entry of LT-HSCs under steady-state conditions and thereby Nrf2 deficiency prevents LT-HSCs from proliferation-induced exhaustion.
These results show that KEAP1-NRF2 system plays an important role in the stress-responsive proliferation and differentiation of quiescent HSCs.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.