Abstract
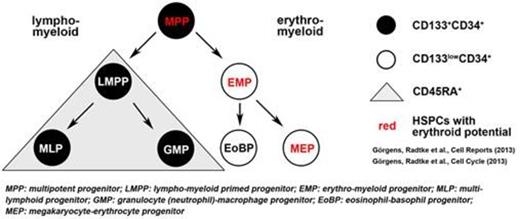
The use of CD133 has recently been described to further characterize human hematopoietic stem/progenitor cells (HSPCs). This led to a revised model of hematopoiesis, in which hematopoietic stem cells and multipotent progenitors (HSCs/MPPs: CD34+ CD133+ CD45RA-) divide asymmetrically giving rise to lympho-myeloid progenitors (LMPPs: CD34+ CD133+ CD45RA+) and erythro-myeloid progenitors (EMPs: CD34+ CD133low CD45RA-). The revised model also suggests, that adult/postnatal multipotent HSCs/MPPs can easily be identified as CD133+ CD34+ cells containing erythroid potentials. Interestingly, previous studies also demonstrated that a substantial fraction of lineage-restricted LMPPs lacking erythro-megakaryocytic lineage-potentials was able to engraft NOD/SCID, therefore questioning the mouse model as a readout for multipotent HSPCs.
Since adult/postnatal and fetal human HSPCs differ in their gene expression profiles, differentiation capabilities and cell surface marker expression, we were interested in studying whether the current revised model of hematopoiesis in adult/postnatal stem cells would also apply to hematopoiesis during embryogenesis. Thus, we comprehensively analyzed the CD133 expression, lineage-relationships and content of multipotent HSPCs in the human fetal liver (FL).
Consistent with findings in adult tissues, CD133 expression allowed for a facile discrimination of distinct HSC/MPP, LMPP and EMP subpopulations in the human FL. We next determined whether the phenotypic findings would also apply to biologic assays. Freshly isolated FL HSPCs were enriched for phenotypical EMPs (30 ± 5%) and HSCs/MPPs (38 ± 3%); the latter population demonstrated extensive proliferation in vitro and also priming towards erythro-myeloid (EMP) differentiation as shown for adult HSPCs. Previous studies have identified the FL with the highest content of HSCs, containing approximately 100/200-fold more HSCs than umbilical cord blood (UCB) and BM respectively. However in contrast to previous reports, the frequency of multipotent CD133+ HSC/MPPs with erythroid differentiation potential in the FL was much lower than expected (0.87 ± 0.16%), with similar frequencies as in UCB and approximately 2-fold higher than in BM. Upon transplantation in neonatal NOD/SCID mice, rapid and stable (29-89%) multi-lineage engraftment in the peripheral blood (PB) was detected after 8-18 weeks showing human B cells, T cells and monocytes. Surprisingly, analysis of engraftment of more primitive human HSPCs in the murine BM revealed that multi-lineage engraftment of human cells was predominantly driven by short-term engrafting LMPPs with very low levels of multipotent human HSPCs in the BM (0.12% of CD34+ cells).
In summary, our data suggests that CD133 expression on FL-HSPCs can be used to identify corresponding functional subpopulations as previously described for adult human stem cell sources and UCB. Surprisingly however, the frequency of multipotent CD133+ HSC/MPPs was substantially lower than expected and, similar to findings with UCB, engraftment of FL-derived CD34+ HSPCs in mice was mostly driven by committed progenitor cells (LMPPs). These data thus challenge the general assumption that the human FL contains greater amounts of multipotent HSCs/MPPs than adult stem cell sources and the use of the SRC assay as a readout for HSCs.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.