Abstract
Background: Endothelial activation and dysfunction play critical roles in vaso-occlusive crises and vasculopathy in sickle cell disease (SCD). However, it remains unclear how the myriad of cellular and biomolecular interactions that occur in SCD directly affect endothelial cell activation and injury, due largely in part to the lack of robust in vitro models for studying these complex biophysical processes. To this end, we engineered the first perfusable hydrogel-based microfluidic device comprised of "endothelialized" channels at the microvascular sizescale. Unlike typical microfluidic devices, which are silicone(PDMS)-based, this device is comprised of a collagen-based hydrogel that is not only more physiologic but also enables real-time monitoring of the endothelium permeability while allowing the user to tightly control hemodynamic conditions and the cellular and molecular components of the perfusate. Interestingly, in this system, upon removal of injurious stimuli, the endothelial cells are able to "self-heal" after injury and fully establish their barrier function. Using this system, we first tested whether interaction of SCD patient RBCs with endothelium under flow can directly cause endothelial permeability. We then studied how shear stress promotes endothelial cell activation and injury caused by free hemin, a byproduct of hemolysis in SCD.
Results and Discussions: After seeding into the hydrogel-based device, human endothelial cells formed a monolayer that covered the entire inner surface of the microchannels and can be maintained for >1 month under flow conditions. Establishing that cultured endothelial cells are functional in this system, the cells appropriately formed continuous adherens junctions under flow as indicated by VE-cadherin staining (Figure 1A) and also deposited their own subendothelial extracellular matrices, including collagen IV (Figure 1B) and laminin (Figure 1C). Finally, perfusion of fluorescently-tagged albumin (BSA) sufficient endothelial barrier function of our system as all fluorescence signal was contained within the "vascular" space (Figure 1D and 1E).
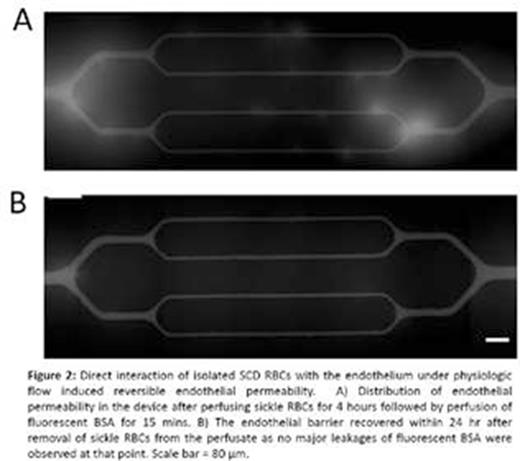
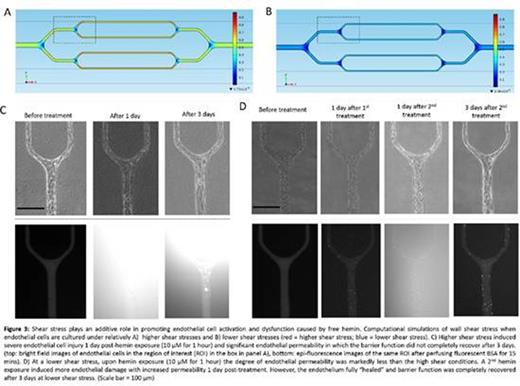
RBCs isolated from SCD patients were perfused into the endothelialized channels for 4 hours. Strikingly, the direct interaction of the perfused SCD RBCs with the engineered endothelium, in and of itself, was sufficient to induce endothelial permeability (Figure 2A), a phenomenon that did not occur with perfusion of control RBCs isolated from healthy volunteers. Interestingly, impermeability was reestablished as the endothelium "healed" 1 day post-interaction with patient RBCs (Figure 2B). We then perfused hemin in the channels under two different flow rates and the flow velocity profile, wall shear stress, shear rate, and pressure were characterized using COMSOL (Figure 3A and 3B). Interestingly, 1 hour of hemin exposure (10 µM) at higher shear stress compared to lower shear stress not only caused increased endothelial permeability and loss of endothelial cells at 1 day post-treatment, but also dampened the "healing" of injured endothelial cells and reestablishment of impermeability after removal of hemin from the system (Figure 3C and D).
Conclusions and on-going work: Our physiologic hydrogel-based microvasculature-on-a-chip system enables investigation of how cell/molecular interactions directly affect endothelial permeability in real time and represents a milestone in the use of microfluidic devices for SCD research. In addition, the "self-healing" capability mimics the in vivo microvasculature and is a unique capability of this system as compared to current in vitro models. With this novel system, we determined that RBC-endothelial interactions under physiologic flow are sufficient to induce endothelial barrier dysfunction. We also demonstrated the additive role of hemodynamics in promoting hemin-induced endothelial activation and dysfunction. Ongoing work investigating the mechanisms of our observations will unveil insight into SCD pathogenesis and our system can be applied to other vascular diseases as well.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.