Abstract
Background
Ixazomib is the first orally administered PI to be studied in the clinic. The feasibility of combining a PI with cyclophosphamide and dexamethasone has been demonstrated with the PI bortezomib (Reeder et al Leukemia 2009; Mai et al Leukemia 2015). This open-label, multicenter, phase 2 study is evaluating the all-oral triplet combination of ICd, with two different doses of cyclophosphamide, as a 12-month induction therapy in previously untreated, transplant-ineligible pts with NDMM, and is the first study to assess ICd for the frontline treatment of MM.
Methods
Adult pts with previously untreated, symptomatic NDMM who were ineligible for stem cell transplantation due to age and/or comorbidities, had ECOG PS 0-2, and adequate hematologic, hepatic, and renal function, were included. Pts were randomized 1:1 to receive up to 13 x 28-day cycles of induction therapy with ixazomib 4.0 mg PO on days 1, 8, and 15, plus cyclophosphamide 300 mg/m2 (ICd-300 arm) or 400 mg/m2 (ICd-400 arm) PO on days 1, 8, and 15, plus dexamethasone 40 mg PO (20 mg in pts aged >75 years) on days 1, 8, 15, and 22. A safety lead-in evaluation of dose-limiting toxicities (DLTs) was performed in 6 evaluable pts in each arm after cycle 1. The primary endpoint was the combined rate of complete response plus very good partial response (CR+VGPR). Secondary endpoints included overall response rate (ORR; CR+VGPR+ partial response [PR]) and safety (adverse events [AEs]). Response was investigator-assessed at the end of every cycle per IMWG criteria. Sample size (n=70) was determined to provide 80% power for the primary endpoint of CR+VGPR rate (1-sided alpha=0.10). Here we present a preliminary analysis of data post-induction (data cut-off: July 1, 2015).
Results
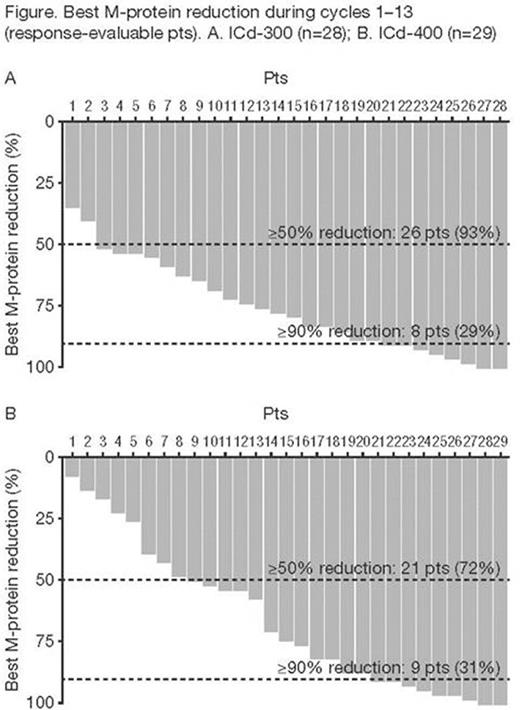
70 pts were randomized (36 to ICd-300, 34 to ICd-400): median age 72.5 and 75.5 years; 42% and 53% male; 64% and 59% ISS stage II/III (92% and 79% Durie-Salmon stage II/III), respectively. Mean duration of follow-up was 7.0 months in both arms. Response data are summarized in the Table. Best unconfirmed CR+VGPR rates across all 13 cycles were 27% (ICd-300) and 23% (ICd-400); ORRs were 80% and 73%. Best M-protein reductions are shown in the Figure. Twelve pts (6 ICd-300; 6 ICd-400) were DLT-evaluable; no DLTs were observed in either arm. Pts received a median of 6.0 (1-13) and 6.5 (1-13) cycles in the ICd-300 and ICd-400 arms, respectively. Mean ixazomib relative dose intensity was 90.7% in the ICd-300 arm and 89.8% in the ICd-400 arm. Across all 13 cycles of treatment (ICd-300 and ICd-400, respectively), rates of Gr ≥3 AEs were 53% and 62%, serious AEs 33% and 53%, AEs leading to dose reduction in any study drug 17% and 21%, discontinuation of all study drugs due to AEs 14% and 12%, and on-treatment deaths 2 pts (cardiac arrest; upper gastrointestinal hemorrhage) and 1 pt (pneumonia) which were not deemed as treatment-related. In the ICd-300 and ICd-400 arms, respectively, rates of anti-emetic use were 36% and 44% (8% and 18% for AEs) and G-CSF use 11% and 50% (11% and 35% for AEs); erythropoietin was used in only 1 pt (ICd-300 arm). Thrombocytopenia events occurred in 5 pts (no Gr ≥3) in the ICd-300 arm and 4 pts (3 Gr ≥3) in the ICd-400 arm. Most common AEs (>15% all pts) were anemia (19% and 29%), neutropenia (17% and 32%), nausea (14% and 24%), peripheral neuropathy (PN; 17% and 21%), diarrhea (19% and 15%), vomiting (14% and 21%), constipation (17% and 15%), and fatigue (14% and 18%). Most common Gr ≥3 AEs were neutropenia (14% and 32%), anemia (11% and 15%), and pneumonia (8% and 9%); no Gr ≥3 PN was observed.
Conclusion
These preliminary data suggest that the all-oral triplet combination of ICd is tolerable in transplant-ineligible pts with NDMM, with a manageable toxicity profile in line with that previously seen with ixazomib and with manageable myelosuppression. Comparably high response rates were reported in both the ICd-300 and ICd-400 arms. Toxicity rates appeared higher with ICd-400, suggesting that ICd-300 may be a more preferable regimen for elderly NDMM pts. Updated data, including long-term outcomes after additional follow-up will be presented at the meeting.
Best unconfirmed response by IMWG criteria during cycles 1-13 (response-evaluable pts)
| Response, n (%) . | ICd-300 (n=30) . | ICd-400 (n=30) . |
|---|---|---|
| CR | 3 (10) | 3 (10) |
| PR | 21 (70) | 19 (63) |
| VGPR | 5 (17) | 4 (13) |
| CR+VGPR | 8 (27) | 7 (23) |
| ORR (CR+VGPR+PR) | 24 (80) | 22 (73) |
| SD | 6 (20) | 8 (27) |
| Response, n (%) . | ICd-300 (n=30) . | ICd-400 (n=30) . |
|---|---|---|
| CR | 3 (10) | 3 (10) |
| PR | 21 (70) | 19 (63) |
| VGPR | 5 (17) | 4 (13) |
| CR+VGPR | 8 (27) | 7 (23) |
| ORR (CR+VGPR+PR) | 24 (80) | 22 (73) |
| SD | 6 (20) | 8 (27) |
SD, stable disease
Dimopoulos:Amgen: Honoraria; Onyx: Honoraria; Celgene: Honoraria; Genesis: Honoraria; Novartis: Honoraria; Janssen-Cilag: Honoraria; Janssen: Honoraria. Off Label Use: Investigational proteasome inhibitor ixazomib in combination with cyclophosphamide and low-dose dexamethasone for patients with newly diagnosed multiple myeloma who are transplant-ineligible.. Nahi:Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Byrne:Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Labotka:Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Hui:Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Teng:Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment.
Author notes
Asterisk with author names denotes non-ASH members.