Abstract
Background: Diffuse large B-cell lymphoma (DLBCL) is curable for the majority of patients treated with anthracycline based immunochemotherapy (IC). However, up to 40% of patients will relapse or require retreatment of DLBCL and outcomes are poor in this setting. Here we examine the incidence, treatment patterns and outcomes of relapsed DLBCL in the R-CHOP era.
Methods: Patients were prospectively enrolled in the University of Iowa / Mayo Clinic SPORE Molecular Epidemiology Resource (MER) within 9 months of diagnosis and followed for relapse, retreatment, and death. Clinical management at diagnosis and subsequent therapies were per treating physician. This analysis includes patients with DLBCL or primary mediastinal B-cell lymphoma (PMBCL) who underwent front-line anthracycline based IC; patients with primary CNS lymphoma or PTLD were excluded. All relapse and re-treatments were verified by medical record review. Response to front-line therapy was retrospectively classified per 2007 Revised Response Criteria for Malignant Lymphoma from available clinical and radiology records. Unplanned consolidative radiation (RT) without biopsy proven disease after achieving PR from IC (N=21) was not classified as a relapse.
Results: 1039 patients with newly diagnosed DLBCL or PMBCL and treated with IC were enrolled in the MER from 2002-2012. Median age at diagnosis was 62 years (range 18-92) and 577 patients (56%) were male. 647 patients (63%) had stage III/IV disease and IPI at diagnosis was 0-1 in 350 patients (34%), 2 in 305 patients (29%), 3 in 250 patients (24%) and 4-5 in 134 patients (13%).
At a median follow-up of 59 months (range 1-148), 258 patients had relapse or retreatment of DLBCL of which 184 (71%) died. Incidence of relapse was 21.7% (95% CI: 19.3%-24.4%) at 2 years and 25.5% (95% CI: 22.9%-28.5%) at 5 years. In addition, the incidence of lymphoma related death without documented relapse or retreatment was 4.7% (95% CI: 3.6%-6.2%) at 2 years. At first relapse, 174 patients (67% of relapsed) received platinum based salvage therapy with 90 (52%) subsequently proceeding to autologous stem cell transplant (ASCT). 22 patients received CNS directed systemic therapy at relapse with 9 (41%) proceeding to transplant, and 43 received non-platinum-based salvage systemic therapy with 7 proceeding to transplant (17%), 15 patients received RT only as 2nd line therapy, and 4 were untreated. At a median follow-up of 56 months (range 6-121) post-transplant, 39 of 107 patients who underwent transplant remain in remission with a 2-year post-transplant progression-free survival of 45% (95% CI 37%-56%).
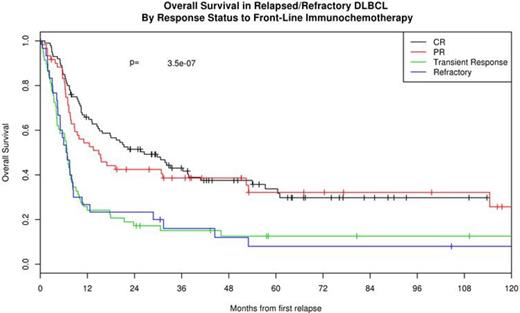
Response to front-line IC was predictive of post-relapse outcome. Survival post-relapse was superior in the 162 patients with responsive disease (CR or PR) at the end of front-line IC (median OS 21.0 months) compared to the 88 patients who had stable or progressive disease (median OS 6.8 months, HR = 2.33, 95% CI: 1.73-3.14 p<0.0001). Transient response in midst of front-line IC was similar to no response. Patients achieving a CR or PR to front-line IC were more likely to proceed to ASCT at relapse (55%) compared to patients with either SD or PD at the end of front-line IC (25% and 17% respectively, p<0.0001). Other factors associated with poor survival at first relapse were relapse within 12 months of diagnosis (HR = 2.24, 95% CI: 1.57-3.18, p<0.0001), IPI at diagnosis of 3-5 (HR=1.51, 95% CI: 1.13-2.03, p=0.0058), and age > 60 (HR =1.51, 95% CI: 1.12-2.03, p=0.0064). There was no difference in survival at first relapse by cell of origin (HR = 1.13, 95% CI: 0.74-1.72, p=0.59).
Conclusions: Most patients undergo therapy after relapsed/refractory DLBCL but only one-third receive ASCT. Outcomes following all treatments for relapsed/refractory DLBCL remain poor. Factors associated with adverse outcomes include refractory to front-line therapy, early relapse, baseline IPI and advanced age. These outcomes provide relevant historical control for the many novel agents being tested in this unmet need.
Farooq:Kite Pharma: Research Funding. Maurer:Kite Pharma: Research Funding. Cerhan:Kite Pharma: Research Funding. Link:Genentech: Consultancy, Research Funding; Kite Pharma: Research Funding. Thompson:Kite Pharma: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.