Abstract
Early therapeutic decision-making is crucial in patients with higher-risk MDS where median survival is only around one year. Azacitidine prolongs survival for these patients (Fenaux et al, Lancet Oncology 2009) but clinically relevant biomarkers remain to be identified. We evaluated retrospectively, the impact of clinical parameters and mutational profiles in 134 consecutive patients treated with a median number of 7 cycles of Azacitidine (range 1-45), in accordance to European guidelines. The vast majority (n=114) had higher-risk disease i.e. MDS with IPSS int-2 or high, AML with multilinear dysplasia and 20-30% blasts or CMML-II. We combined an initial cohort from Karolinska University Hospital (n=89) with a validating cohort from King's College Hospital, London (n=45). Studied endpoints were response, as defined by the IWG criteria, and survival. Since prolonged survival is the main goal for this cohort of patients, we believe that survival is the most relevant endpoint, supported by the fact that even non-responding patients have a survival benefit from Azacitidine (Gore et al, Haematologica, 2013).
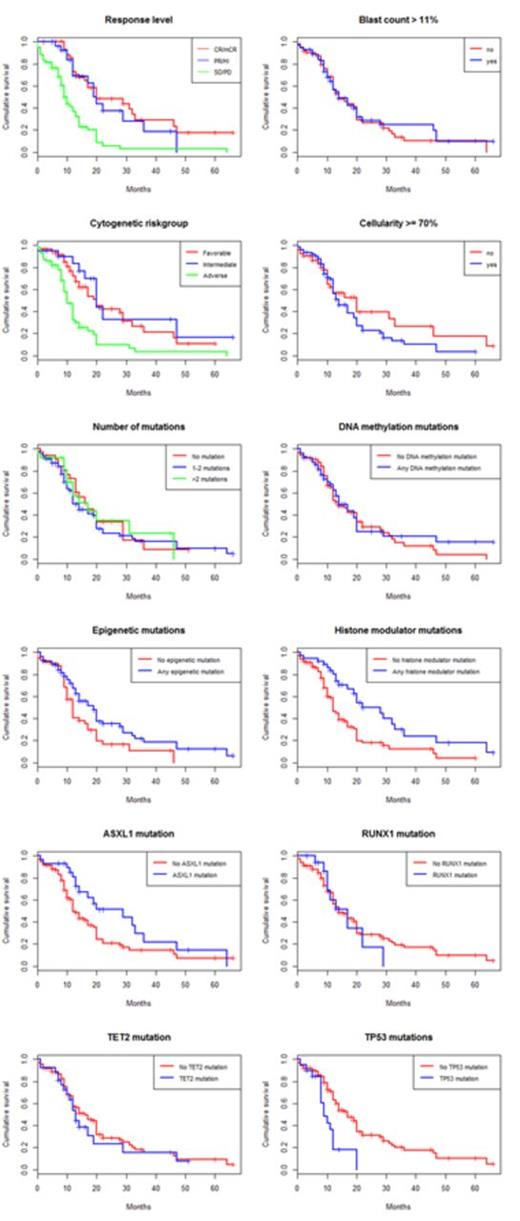
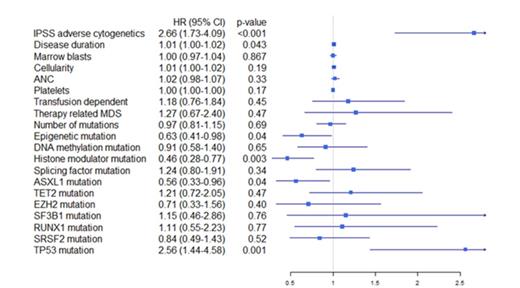
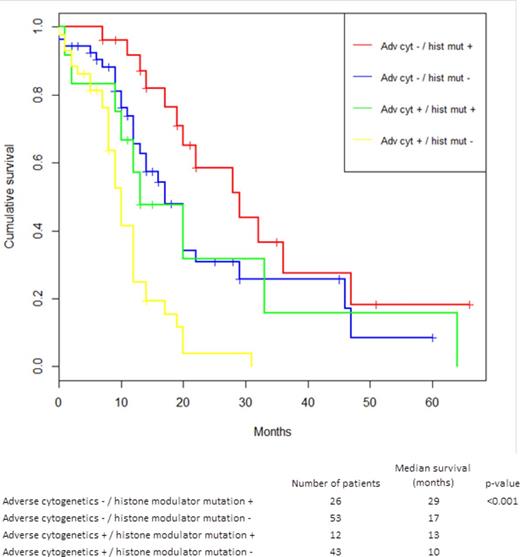
While neither clinical parameters nor mutations had a significant impact on response rate, both karyotype and mutational profile were strongly associated with survival from the start of treatment, see Table 1 and Figure 1-2. IPSS high-risk cytogenetics was negatively associated with survival (median 20 vs 10 months; p<0.001), whereas mutations in histone modulators (ASXL1, EZH2, MLL) were associated with prolonged survival (22 vs 12 months, p=0.001). This positive association was present in both cohorts and remained highly significant in the multivariate cox model. Importantly, patients with mutations in histone modulators lacking high-risk cytogenetics showed a survival of 29 months (response rate 73%) compared to 10 months (response rate 49%) in patients with the opposite pattern, see Figure 3. While TP53 was negatively associated with survival in the univariate analysis, neither RUNX1-mutations nor the number of mutations, previously reported as negative prognostic markers, appeared to influence survival in this cohort. In contrast, disease duration and cellularity showed a weak negative correlation with survival. We propose a model combining histone modulator mutational screening with cytogenetics in the clinical decision-making process for higher-risk MDS eligible for treatment with Azacitidine.
Variables associated with survival. Univariate analyses used the log-rank test. The cox model included all listed variables except response rate in a multivariate analyses.
| . | Estimated median survival (months) . | Univariate p-value . | Cox regression p-value . | Hazard ratio (95% CI) . |
|---|---|---|---|---|
| Response rate: CR / mCR vs PR/HI vs SD/PD | 20 vs 20 vs 10 | <0.001 | ||
| IPSS cytogenetic risk group: Favorable vs Int vs Adverse | 20 vs 20 vs 10 | <0.001 | <0.001* | 3.00 (1.9-4.7) |
| Disease duration ≥ 4 months: Yes vs No | 14 vs 17 | 0.44 | 0.05** | 1.01 (1.00-1.02) |
| Marrow blasts ≥ 11%: Yes vs No | 14 vs 14 | 0.7 | ||
| Cellularity ≥ 70%: Yes vs No | 14 vs 20 | 0.2 | 0.02 | 1.013 (1.002-1.023)** |
| ANC ≥ 1.3: Yes vs No | 14 vs 17 | 0.32 | ||
| Platelets ≥ 60: Yes vs No | 17 vs 12 | 0.07 | ||
| Transfusion dependent: Yes vs No | 13 vs 17 | 0.43 | ||
| Therapy related: Yes vs No | 17 vs 14 | 0.44 | ||
| Number of mutations: 0 vs 1 vs ≥ 2 | 17 vs 12 vs 17 | 0.64 | ||
| Epigenetic mutation: Yes vs No | 19 vs 12 | 0.03 | ||
| DNA methylation mutation: Yes vs No | 14 vs 14 | 0.64 | ||
| Histone modulator mutation: Yes vs No | 22 vs 12 | 0.001 | 0.007 | 0.499 (0.3-0.83) |
| Splicing factor mutation: Yes vs No | 13 vs 17 | 0.31 | ||
| ASXL1 mutation: Yes vs No | 29 vs 12 | 0.03 | ||
| TET2 mutation: Yes vs No | 13 vs 16 | 0.45 | ||
| EZH2 mutation: Yes vs No | 20 vs 14 | 0.37 | ||
| SF3B1 mutation: Yes vs No | 13 vs 16 | 0.35 | ||
| RUNX1 mutation: Yes vs No | 17 vs 14 | 0.76 | ||
| SRSF2 mutation: Yes vs No | 20 vs 14 | 0.5 | ||
| TP53 mutation: Yes vs No | 9 vs 17 | <0.001 |
| . | Estimated median survival (months) . | Univariate p-value . | Cox regression p-value . | Hazard ratio (95% CI) . |
|---|---|---|---|---|
| Response rate: CR / mCR vs PR/HI vs SD/PD | 20 vs 20 vs 10 | <0.001 | ||
| IPSS cytogenetic risk group: Favorable vs Int vs Adverse | 20 vs 20 vs 10 | <0.001 | <0.001* | 3.00 (1.9-4.7) |
| Disease duration ≥ 4 months: Yes vs No | 14 vs 17 | 0.44 | 0.05** | 1.01 (1.00-1.02) |
| Marrow blasts ≥ 11%: Yes vs No | 14 vs 14 | 0.7 | ||
| Cellularity ≥ 70%: Yes vs No | 14 vs 20 | 0.2 | 0.02 | 1.013 (1.002-1.023)** |
| ANC ≥ 1.3: Yes vs No | 14 vs 17 | 0.32 | ||
| Platelets ≥ 60: Yes vs No | 17 vs 12 | 0.07 | ||
| Transfusion dependent: Yes vs No | 13 vs 17 | 0.43 | ||
| Therapy related: Yes vs No | 17 vs 14 | 0.44 | ||
| Number of mutations: 0 vs 1 vs ≥ 2 | 17 vs 12 vs 17 | 0.64 | ||
| Epigenetic mutation: Yes vs No | 19 vs 12 | 0.03 | ||
| DNA methylation mutation: Yes vs No | 14 vs 14 | 0.64 | ||
| Histone modulator mutation: Yes vs No | 22 vs 12 | 0.001 | 0.007 | 0.499 (0.3-0.83) |
| Splicing factor mutation: Yes vs No | 13 vs 17 | 0.31 | ||
| ASXL1 mutation: Yes vs No | 29 vs 12 | 0.03 | ||
| TET2 mutation: Yes vs No | 13 vs 16 | 0.45 | ||
| EZH2 mutation: Yes vs No | 20 vs 14 | 0.37 | ||
| SF3B1 mutation: Yes vs No | 13 vs 16 | 0.35 | ||
| RUNX1 mutation: Yes vs No | 17 vs 14 | 0.76 | ||
| SRSF2 mutation: Yes vs No | 20 vs 14 | 0.5 | ||
| TP53 mutation: Yes vs No | 9 vs 17 | <0.001 |
*Comparing adverse cytogenetics vs the other groups. ** Disease duration, marrow blasts, cellularity, ANC and TPK were analyzed as a continuous variable in the cox model
Kaplan-Meier estimated survival stratified for response and pre-treatment parameters
Kaplan-Meier estimated survival stratified for response and pre-treatment parameters
Forest plot indicating hazard ratio including confidence interval for all pre-treatment variables. The hazard ratios were retrieved using cox univariate regression models for each variable analyzed separately.
Forest plot indicating hazard ratio including confidence interval for all pre-treatment variables. The hazard ratios were retrieved using cox univariate regression models for each variable analyzed separately.
Kaplan-Meier estimated survival stratified for the two dominant predictors in the cox regression model: Adverse cytogenetics and histone modulator mutations
Kaplan-Meier estimated survival stratified for the two dominant predictors in the cox regression model: Adverse cytogenetics and histone modulator mutations
McLornan:Novartis: Research Funding, Speakers Bureau. Jädersten:Celgene: Other: speakers fee. Kulasekararaj:Alexion: Consultancy. Mufti:Celgene: Consultancy, Other: Speakers fee. Hellström-Lindberg:Celgene Corporation: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.