Abstract
Background
Outcomes of haploidentical stem cell transplantation (haploSCT) have improved since the introduction of post-transplantation cyclophosphamide (PTCy) to the extent that survival is comparable to HLA matched donor transplantation. Here, we report the outcomes of 80 patients treated at our institution for de novo acute myeloid leukemia (AML), myelodysplastic syndrome (MDS) and AML arising from MDS treated with melphalan, fludarabine conditioning.
Methods
We retrospectively analyzed data from 80 patients (60 [75%] with de novo AML and 20 [25%] with MDS or MDS/AML who received a haploSCT between 2009 to April 2015. Twenty-nine patients (38%) in total had adverse cytogenetics while 12/18 (66%) of MDS, MDS/AML patients had adverse cytogenetics. Fifty one patients (64%) were in a complete response (CR) at the time of transplant. Thirty-seven patients (46.3%) were conditioned with fludarabine 40 mg/m2 (day -6 to -3), melphalan 100 mg/m2 (day -8), thiotepa 5 mg/kg (day -7), 26 (32.5%) with fludarabine and melphalan 140 mg/m2 and 13 (16%) with fludarabine, melphalan and total body irradiation (TBI) at 200 cGy. Graft vs host disease (GvHD) prophylaxis consisted of PTCy on day +3 and +4 after haploSCT and tacrolimus and mycophenolate for 6 and 3 months, respectively. Donors included children (48%), siblings (41%) and parents (10%). Seventy six patients (95%) received a bone marrow graft and the remaining 4 (5%) received peripheral blood.
Results
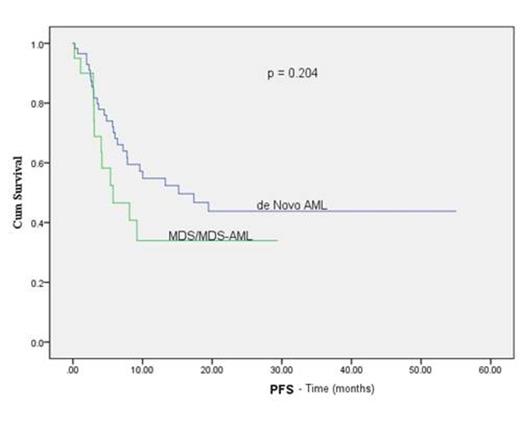
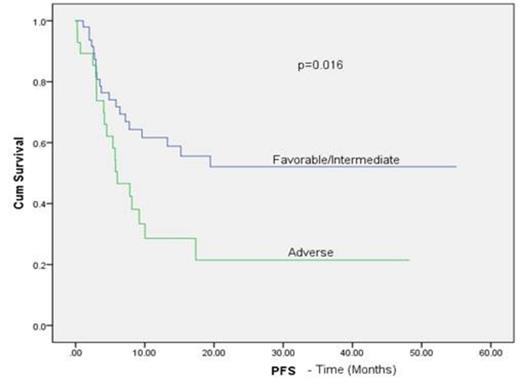
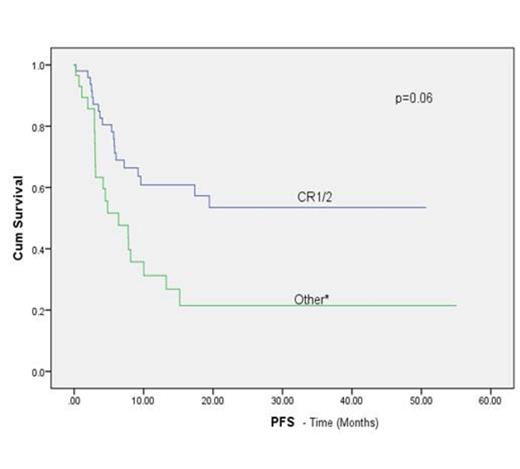
Median age was 50 years (range, 15-69 years), 54% were male. Three patients had early death: 2 from infection and 1 from multiorgan failure. Of the 77 evaluable patients, 76 engrafted (99% primary engraftment). The median times to neutrophil and platelet engraftment were 18 days (range, 6-40 days) and 25 days (range, 10-54 days), respectively. The rates of grade II-IV and III- IV acute GvHD at day 100 were 30% and 3%, respectively. Chronic GvHD occurred in 8 of 60 patients (13%): 3 with extensive and 5 with limited stage. At the last follow-up, 40 patients (50%) were alive and disease-free. Relapse occurred in 17 of the 60 patients with de novo AML (28.3%) and 6 of 20 patients with MDS, MDS/AML (30%). The 1-year and 2-year overall survival rates were 56% and 47.5%, respectively, with 64.6% of patients in CR1 or 2 at the time of transplant and 42.3% of patients with active disease or greater than CR2 alive at 1 year (log rank p= 0.030). On univariate analysis, cytogenetic risk and disease status prior to transplant were found to have a statistically significant influence on progression free survival (PFS) (Figure 1B, C)
All 17 patients with active disease at the time of transplant attained a CR and 7 (41%) remained disease free and alive at the median time of follow-up. The cumulative incidence of non-relapse mortality (NRM) at day 100 and 1 year were 13.8% and 27.4% respectively. Of the 34 deaths; 18 (53%) were due to relapsed disease, 7(20%) infection, 3 GvHD, 5 organ failure and 1 graft failure.
Conclusion
HaploSCT using a fludarabine, melphalan backbone for conditioning is safe and effective not only for patients with de novo AML, but also with MDS and AML arising from MDS. Improvement in relapse rate for patients with high-risk disease and those not in remissions at transplant is warranted.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.