Abstract
* These authors contributed equally to this work
The microvasculature plays a key role in the pathogenesis of sepsis, ARDS, multiorgan dysfunction, and a variety of other human diseases characterized by a pro-coagulant, pro-adhesive endothelial phenotype. The complex interactions that occur at the interface of blood and activated endothelium are difficult to resolve in animal models and challenging to recreate in cell culture systems. While individual processes - e.g., leukocyte adhesion and transmigration - have been extensively studied, development of a well-characterized model integrating the full range of pathogenic processes (coagulation, barrier dysfunction, innate immune system activation, etc.) remains an important unmet goal, which could both elucidate mechanisms of disease and aid in the design and testing of putative therapeutics. To this end, we sought to model an inflamed vascular segment using a Fluxion Bioflux system. 3-dimensional confluent endothelial cell (EC) monolayers were established within fibronectin-coated laminar flow chambers. ECs were flow adapted, activated with TNFa, and then perfused with whole blood (WB) at a variety of shear stresses. Real time fluorescence microscopy allowed continuous monitoring of fibrin deposition and leukocyte and platelet adhesion. The multi-channel format, which allows simultaneous testing of multiple conditions with replicates, proved to be a critical asset, given substantial day-to-day variability. Both fibrin deposition and adhesive events showed dependence on dose (1 vs. 10 ng/mL) and duration (4 vs. 6hr) of TNF activation. Confocal microscopy revealed TNF-dependent, increases in EC expression of ICAM-1, VCAM-1, and tissue factor (TF), as well as suppression of endothelial thrombomodulin (TM). Activation of the coagulation system was completely abrogated by treatment of the EC monolayer with a TF-inhibiting antibody, suggesting a primary role for the extrinsic pathway. Hirudin also limited fibrin deposition when added to whole blood prior to perfusion, although "breakthrough clotting" was seen in some channels. Finally, the role of endothelial TM was investigated in several ways, including by the use of a blocking antibody, which prevents thrombin binding. Treatment of ECs with this antibody markedly increased fibrin deposition, whereas TM/R6.5 scFv, a novel targeted fusion protein therapeutic, which anchors recombinant TM to endothelial ICAM-1, inhibited fibrin deposition upon subsequent infusion of WB. Neither soluble TM (sTM) nor anti-ICAM-1 R6.5 scFv alone had any effect on coagulation when infused in this setting (i.e., prior to WB) and even addition of a large excess of sTM to whole blood was less effective in reducing TNF-dependent fibrin deposition than pre-treatment with the ICAM-targeted TM fusion protein, indicating potential importance of precision drug delivery on the microscale. In summary, the described microfluidic, "endothelialized", whole blood model of an inflamed microvessel may prove useful in interrogating specific aspects of a variety of vascular pathologies and in devising and improving therapeutic interventions.
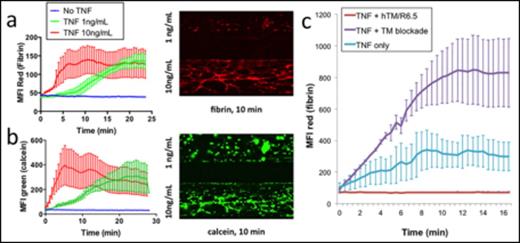
(a) Fibrin deposition and (b) leukocyte and platelet adhesion upon whole blood perfusion of endothelialized microchannels, pre-activated with 1 vs. 10ng/mL of TNF for 6 hours. (c) Role of endothelial TM in TNF-dependent microvessel thrombosis. TM blockade exacerbates fibrin deposition whereas ICAM-targeted TM fusion protein effectively eliminates coagulation.
(a) Fibrin deposition and (b) leukocyte and platelet adhesion upon whole blood perfusion of endothelialized microchannels, pre-activated with 1 vs. 10ng/mL of TNF for 6 hours. (c) Role of endothelial TM in TNF-dependent microvessel thrombosis. TM blockade exacerbates fibrin deposition whereas ICAM-targeted TM fusion protein effectively eliminates coagulation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.