Abstract
Background: The phase 3 A-LONG and Kids A-LONG studies demonstrated the safety and efficacy of recombinant FVIII Fc fusion protein (rFVIIIFc) for control and prevention of bleeding episodes in subjects with severe hemophilia A. The ongoing rFVIIIFc extension study, ASPIRE (Clinicaltrials.gov #NCT01454739), evaluates long-term safety and efficacy of rFVIIIFc in adults/adolescents and children who completed A-LONG and Kids A-LONG, respectively.
Aims: To evaluate the long-term efficacy of rFVIIIFc in subjects with target joints at entry into the parent studies (A-LONG, Kids A-LONG).
Methods: A-LONG and Kids A-LONG were Phase 3, open-label, multicenter studies in males aged ≥12 years (A-LONG) or <12 years (Kids A-LONG) with severe hemophilia A (<1 IU/dL endogenous FVIII activity) and prior FVIII treatment. Subjects in A-LONG were enrolled into 1 of 3 arms: Arm 1, individualized prophylaxis (IP); Arm 2, weekly prophylaxis (WP); or Arm 3, episodic treatment. All Kids A-LONG subjects received rFVIIIFc prophylaxis. Upon completing A-LONG or Kids A-LONG, eligible subjects could enroll in ASPIRE.
There are 4 treatment groups in ASPIRE: IP; WP; modified prophylaxis (MP; for subjects in whom optimal dosing could not be achieved with individualized or weekly prophylaxis); or episodic treatment. Subjects can change treatment groups upon entry or at any point during ASPIRE. Subjects aged ≥12 years can participate in any treatment group; subjects aged <12 years can participate in the individualized and modified prophylaxis groups only. Self-reported prestudy 12-month bleeding history and on-study annualized bleeding rate (ABR), including overall ABR and ABR in target joints present at baseline were evaluated. The current analysis evaluated subjects with ≥1 target joint (major joint with ≥3 bleeding episodes in a 6-month period) at entry into the parent study with available prestudy and on-study data. Outcomes were analyzed over the cumulative duration of the parent study through the ASPIRE interim datacut (January 6, 2014). Subjects were evaluated in each treatment group they participated in, for the duration they were in that treatment group (because of the ability to switch groups, subjects may be included in >1 group).
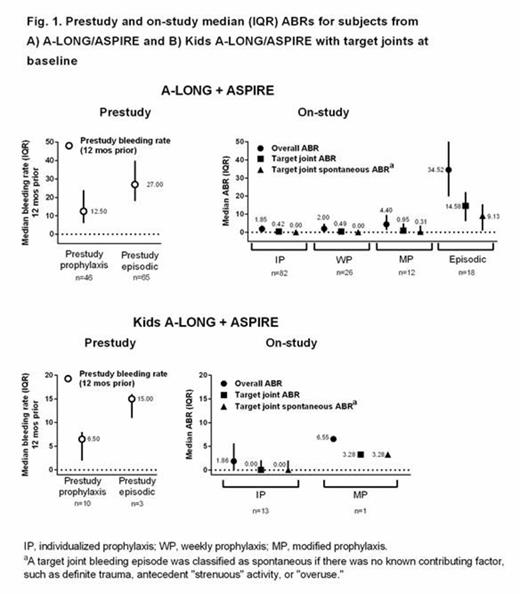
Results: The analyzed population included 111 adult/adolescent and 13 pediatric subjects with target joints at entry into A-LONG and Kids A-LONG, respectively. Median (range) cumulative duration of treatment with rFVIIIFc on A-LONG/ASPIRE was 110 (24-162), 79 (2-110), 80 (45-86), and 33 (16-116) weeks for the IP (n = 82), WP (n = 26), MP (n = 12), and episodic treatment (n = 18) groups, respectively; for Kids A-LONG/ASPIRE, median (range) duration was 51 (22-58) and 16 weeks for the IP (n = 13) and MP (n = 1) prophylaxis groups, respectively. Among 111 A-LONG subjects, there were 287 target joints at baseline (located in the elbow [n = 100; 34.8%], ankle [n = 92; 32.1%], knee [n = 63; 22.0%], shoulder [n = 17; 5.9%], wrist [n = 9; 3.1%], and hip [n = 6; 2.1%]). Among 13 Kids A-LONG subjects, there were 15 target joints at baseline (located in the ankle [n = 10; 66.7%], elbow [n = 4; 26.7%], and knee [n = 1; 6.7%]).
In subjects with target joints at baseline, median on-study overall ABRs with rFVIIIFc prophylaxis for adults/adolescents (Fig. 1A) and children (Fig. 1B) tended to be lower than prestudy bleeding rates. On-study, both provoked and spontaneous target joint median ABRs were low.
A total of 47.6%, 42.3%, and 41.7% of subjects in the IP, WP, and WP groups, respectively, had no target joint bleeding episodes during A-LONG/ASPIRE; for Kids A-LONG subjects, 53.8% of subjects in the IP group had no target joint bleeding episodes on-study. Median average total weekly rFVIIIFc prophylactic doses and median dosing intervals during A-LONG/ASPIRE and Kids A-LONG/ASPIRE for subjects with target joints at baseline were similar to those for the overall study populations.
Summary/Conclusion: For subjects with target joints at baseline, efficacy data from the phase 3 and extension trials suggest that long-term use of extended half-life rFVIIIFc prophylaxis is effective for prevention of target joint bleeding.
Kerlin:Bayer Healthcare US and Novo Nordisk: Membership on an entity's Board of Directors or advisory committees; CSL Behring: Research Funding. Kulkarni:Baxter: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pfizer: Membership on an entity's Board of Directors or advisory committees; Kedrion: Membership on an entity's Board of Directors or advisory committees; BPL: Membership on an entity's Board of Directors or advisory committees; Biogen: Research Funding, Speakers Bureau. Nolan:Biogen and Sobi: Research Funding. Wang:Biogen: Membership on an entity's Board of Directors or advisory committees; CSL Behring: Membership on an entity's Board of Directors or advisory committees; Baxalta: Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees. Pasi:Octapharma: Research Funding; Biogen, Octapharma, Genzyme, and Pfizer: Consultancy, Honoraria. Liesner:Sobi: Membership on an entity's Board of Directors or advisory committees, Research Funding. Brown:Biogen, Novo Nordisk, Baxter, and Pfizer: Other: Sponsorship to meeting. Hanabusa:Novo Nordisk, Baxalta, Bayer, Pfizer, Biogen, and KaketsuKen: Honoraria; Novo Nordisk, Baxalta, KaketsuKen, and Biogen: Membership on an entity's Board of Directors or advisory committees. Tsao:Biogen: Employment, Equity Ownership. Allen:Biogen: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.