Abstract
Introduction: Delayed hematopoietic recovery and increased risk of transplant related mortality (TRM) continue to be major risk factors for patients undergoing myeloablative cord blood transplant (CBT). To overcome this issue, our group has developed methods for the ex vivo expansion of cord blood (CB) derived hematopoietic stem and progenitor cells (HSPCs) using an engineered Delta-1 Notch ligand. This expanded CB HSPC product has now been developed for use as a cryopreserved, universal donor (non-HLA matched), off-the-shelf (OTS) cell therapy to be infused as an adjuvant graft in CBT recipients. We have previously reported the promising clinical outcomes observed after infusion of the expanded OTS cell product after myeloablative CBT for faster neutrophil and platelet recovery, reduced TRM and grade III-IV acute GVHD and higher overall survival rate in comparison to the conventional CBT patients1. Based on these results, a multicenter Phase II randomized clinical trial is now underway. Herein, we sought to identify factors that contributed to the improved outcome in these patients by defining the patterns of early hematopoietic recovery in the peripheral blood of patients during the first four weeks after myeloablative CBT.
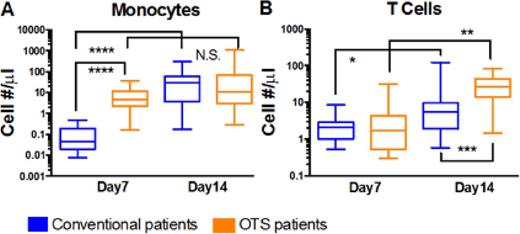
Method: Between February 2013 and June 2015, 27 patients were enrolled in this study to assess the efficacy of our OTS product as an adjuvant graft to improve hematopoietic reconstitution and reduce regimen related toxicities in recipients of myeloablative CBT. Fourteen of the 27 patients were randomized to receive the OTS product. The frequencies of monocytes, T cells and NK cells (defined as CD14+, CD3+ and CD56+ respectively) within the freshly isolated peripheral blood mononuclear cells were determined by real-time immunophenotyping using multi-color flow cytometry on days 7, 14, 21, and 28 following CBT 2.
Results: Strikingly, on day 7 the median absolute number of monocytes was ~100-fold higher in the OTS group as compared to the conventional group (4.7 cells/μL, 95% CI: 2.0 - 13 cells/μL vs 0.04 cells/µL, 95% CI: 0.01- 0.2 cells/μL), however by day 14 the absolute number of monocytes was similar in the two groups. Chimerism studies further confirmed that on day 7 nearly all (95-100%) of the circulating myeloid cells were derived from the OTS graft, whereas on day 14 the CD14 fraction was predominantly from the unmanipulated conventional graft. Furthermore, we anticipated that infusion of a non-HLA matched cell product would induce enhanced alloreactivity of the unmanipulated CB unit in vivo. Thus, we set out to analyze the frequency and composition of the donor derived peripheral blood T cells in both groups. We did not observe any differences in the absolute number of T cells present on day 7, but on day 14, we documented a robust expansion of circulating T cell in OTS patients (median=26 cells/µL, 95% CI: 12 - 50 cells/μL in the OTS group vs 5.4 cells/µL, 95% CI: 1.7 - 12 cells/μL, in the conventional group, p=0.0015). T cell subset analysis revealed that it is CD8+ T cells that are the primary contributor to the observed total T cell burst on day 14, with an absolute median number of 20.2/μL (OTS) vs 1.29/ μL (conventional), CI 95%: 10.7 - 36.6 vs 0.53 - 8.75, p = 0.0003. Additionally, a small, ~3- fold, increase in numbers of CD4+ T cells from day 7 to 14 was seen in the OTS patients but not in the conventional group (p = 0.03), resulting in a significantly lower median day 14 CD4:CD8 ratio in OTS patients than in conventional patients (0.17 vs 1.4, 95% CI: 0.1- 0.5 vs 0.4 - 3.9, p =0.004). Moreover, the analysis of NK cells at any time point and monocytes and T cells on day 21 and 28 did not yield any statistically significant changes in patients in each group
Conclusion: Our findings show that patients receiving a non-HLA matched OTS product display a distinct pattern of early immune cell recovery characterized by an early monocyte burst on day 7 as "myeloid bridge" and a CD8 T cell burst on day 14. Better understanding of how these observations could impact the overall clinical outcomes in myeloablative CBT recipients will potentially expand the future applications of the non-HLA matched OTS product. SHAPE \* MERGEFORMAT
1. Milano F. et al. Blood. 2014;124(21):46.
2. Li J. et al. Blood. 2014;124(21):3897.
Delaney:Biolife Solutions: Membership on an entity's Board of Directors or advisory committees; medac: Research Funding; Novartis: Other: Chair, DSMB.
Author notes
Asterisk with author names denotes non-ASH members.