Abstract
Introduction
The extent of systemic iron overload (SIO), quantified by magnetic resonance imaging (MRI), has been associated with adverse outcome in some studies in MDS and AML patients undergoing allogeneic stem cell transplantation (allo-SCT), whereas others were unable to demonstrate a significant impact. It has been hypothesized that the release of reactive iron species such as labile plasma iron (LPI) during the transplant procedure mediates iron-associated cellular toxicity by catalyzing the generation of oxygen radicals and fostering the growth of microbial agents. The association between SIO, the occurrence of LPI and the outcome after allo-SCT has not been prospectively studied so far.
Patients, Material and Methods
This was a Geman-Austrian prospective multicenter observational trial in 133 patients with AML or MDS undergoing allo-SCT between 2013 and 2015 (NCT01746147). Inclusion criteria were either having a ferritin above 500 ng/ml or having received more than 10 red blood cell concentrates. Liver iron content (LIC) was determined by MRI prior to and on day +100 and day +360 after allo-SCT. Enhanced labile plasma iron (eLPI) was measured using the Ferros eLPI Kit (Afferix) prior to, during and after conditioning and an eLPI above 0.4 was defined as positive.
Results
At the time of analysis 21 MDS and 90 AML patients were evaluable for LIC. The median age of the cohort was 61 years (range: 21 to 75 years) and the majority (80.2 %) received reduced intensity conditioning regimens. Median LIC prior to conditioning was 110 µmol/g and 45.9 % had a LIC above the pre-specified threshold of 125 µmol/g (7 mg/g) indicating SIO.
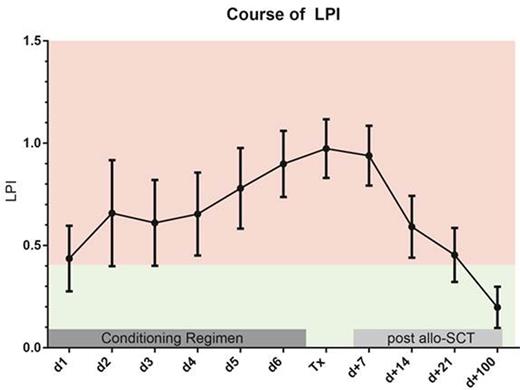
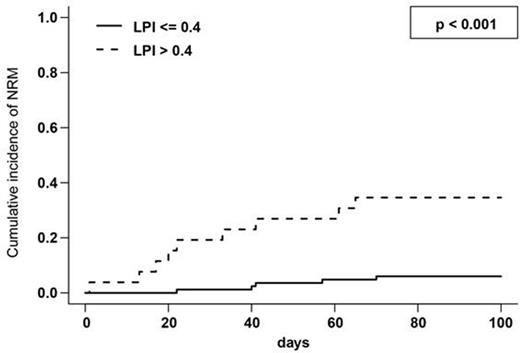
A LIC >=125 µmol/g was associated with a significantly increased cumulative incidence (CI) of early (day +100) NRM (19.8 % vs. 6.8 % p = 0.034), thus confirming our previous observations (Wemke et al. ClinCancRes 2012). Prior to the initiation of the conditioning regimen positive eLPI levels were found in 26 of 109 evaluable patients. A significant correlation between LIC and pre-conditioning eLPI (Pearson's correlation coefficient: 0.470; p < 0.001) was noted. In fact, the median LIC in patients with a pre-conditioning eLPI > 0.4 was 190 µmol compared to 100 µmol/g in patients below this threshold (p < 0.001). Mean eLPI levels increased continuously during the course of the conditioning regimen and then gradually decreased starting on day +7, while most patients had negative eLPI levels by day +100 after allo-SCT (Figure 1). The presence of an eLPI above 0.4 prior to the initiation of the conditioning regimen was strongly associated with an increased early NRM (CI at day +100: 34.6 % vs. 6.0 % p < 0.001, Figure 2) and this association was confirmed in a multivariate analysis incorporating other factors known to predict for NRM (HR 7.0; 95% confidence interval: 2.076 to 23.91; p = 0.002). Of note, patients remaining LPI positive at day +14 also had a significantly increased NRM (19.0 % vs. 4.9 % p = 0.025), which also held true, when the analysis was restricted to patients being LPI negative prior to conditioning (12.5 % vs. 0.0 % p = 0.013).
Patients having an eLPI above 0.4 prior to conditioning had a slightly higher CI of bacterial infections during the course of transplant (CI at day +100: 88.5 % vs. 83.3 %, p = 0.023). There was no association between a positive pre-conditioning eLPI and the occurrence of acute graft versus host disease of grade 2 or higher (CI: 39.1 % vs. 38.6 %).
Conclusions
The results of the prospective ALLIVE trial confirm recent single center observations that SIO prior to allo-SCT is associated with an increased mortality in AML and MDS patients. Given the fact that a positive eLPI prior to the initiation of the conditioning regimen and the persistence of positive eLPI levels after transplantation are strongly predictive for adverse outcome, it is reasonable to believe that reactive iron species are the key pathogenetic mediators in this context. Therefore, clinical trials assessing therapeutic interventions e.g. by peri-transplant iron chelation are warranted.
Wermke:Boehringer: Research Funding; Novartis: Research Funding. Bug:Celgene, Novartis: Research Funding; NordMedica, Boehringer Ingelheim, Gilead: Membership on an entity's Board of Directors or advisory committees; TEVA Oncology, Astellas: Other: Travel Grant. Theurl:Gilead Science: Research Funding. Platzbecker:Novartis: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Boehringer: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.