Abstract
Background:
In a previously published phase-1/2 study, momelotinib (a JAK 1/2 inhibitor) induced 53% anemia, 39% spleen and >50% constitutional symptoms response rates in patients with myelofibrosis (MF) (Pardanani A, et al. Leukemia. 2013;27:1322). The particular study included 100 patients from the Mayo Clinic who experienced 43% spleen and 44% anemia response rates (Pardanani et al. Leukemia. 2015;29:741); CALR and ASXL1 mutations independently predicted spleen but not anemia response. In the current study, we examined the impact of momelotinib therapy on DIPSS-plus- and molecular risk-adjusted survival.
Methods:
The current study represents sponsor-independent analysis of long-term survival among 100 consecutive Mayo Clinic patients who received momelotinib therapy as part of a larger (n =166) phase-1/2 study (NCT00935987). Safety and efficacy data from NCT00935987, as well as protocol design and drug dosing and schedule, have already been formally reported and not reiterated here (Pardanani A, et al. Leukemia. 2013;27:1322; Pardanani A, et al. Leukemia. 2015;29:741; Abdelrahman RA, et al. BJH. 2015;169:77). All statistical analyses considered clinical and laboratory parameters obtained at the time of entry to study. Survival analysis was considered from the date of study entry to date of death or last contact. Cox proportional hazard regression model was used for multivariable analysis of survival. Samples from time of study entry were used for mutation screening. Driver mutations were classified into two prognostically-relevant groups: CALR type 1/type 1-like (favorable) vs all others including CALR type 2/type 2-like, JAK2, MPL and triple-negative (unfavorable).
Results:
100 patients with MF (median age 66 years; 58% males) were treated at the Mayo Clinic between 11/20/09 and 11/10/10; 64 patients had primary MF, 22 post-polycythemia vera MF and 14 post-essential thrombocythemia MF.
Baseline data: DIPSS-plus risk distribution was 63% high, 36% intermediate-2 and 1% intermediate-1 (JCO 2011;29:392); for the purposes of the current analysis, the single patient with intermediate-1 risk disease was included in the intermediate-2 category. 54 (54%) patients displayed abnormal karyotype. 73 (73%) patients harbored JAK2, 16 (16%) CALR, 7 MPL and 4 "triple-negative". Among the 16 CALR -mutated cases, 13 were classified as "type 1/type 1-like". 94 patients were screened for ASXL1 mutations; 41 (44%) were mutated.
Follow-up data was updated in July 2015, providing a minimum follow-up of over 4.5 years since the last patient registration on the protocol. At a median follow-up of 3.2 years, 67 (67%) deaths and 13 (13%) leukemic transformations were documented. Median survival from the time of study entry was 3.2 years and was affected by driver mutation prognostic groups (median survival "not reached" for "favorable" vs 3 years for "unfavorable"; p=0.01), ASXL1 mutational status (median survival 3.9 years in unmutated vs 2.5 years in mutated; p=0.02) and DIPSS-plus risk stratification (median survival 2.6 years in high-risk vs 4 years in intermediate-2 risk; p=0.07).
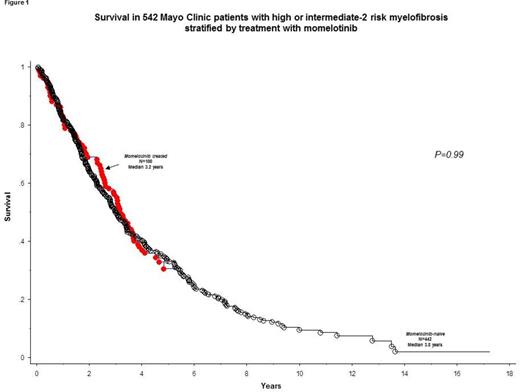
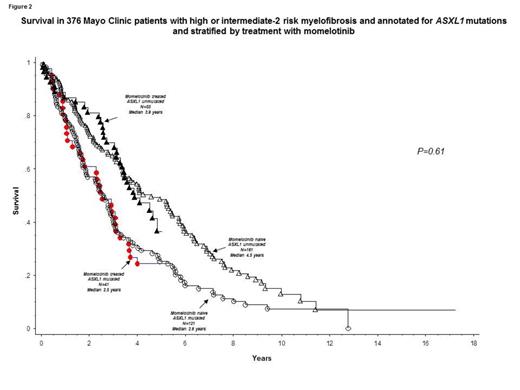
In order to assess the impact of momelotinib on long-term outcome, we compared survival of the study cohort (n =100) with that of 442 consecutive Mayo Clinic patients with high or intermediate-2 risk primary MF (median age 67 years; 66% males). There was no significant difference between this control group of PMF patients versus the momelotinib patient cohort in distribution of age (p=0.56), sex (p=0.12), JAK2/CALR/MPL mutational status (p=0.41) or ASXL1 mutational status (p=0.9); however, the momelotinib cohort had a higher fraction of patients with DIPSS-plus high-risk disease (63% vs 47%; p=0.004). Overall survival was similar between the momelotinib cohort and the control group of PMF patients who were not treated with momelotinib (Figure 1; p=0.99); the difference in survival remained not significant during multivariable analysis that included risk stratification by DIPSS-plus (p=0.32), "favorable" vs "unfavorable" driver mutational status (p=0.78) or ASXL1 mutational status (Figure 2; p=0.61).
Conclusions:
In the current long-term study, we could not demonstrate a survival impact from momelotinib therapy in myelofibrosis. Although adjusted for clinical and molecular risk status, our study is retrospective and cannot be relied upon to discount marginal survival effect.
Al-Kali:Novartis: Research Funding. Pardanani:Stemline: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.