Abstract
Background and Aims: The term Systemic Mastocytosis (SM) identifies a poorly understood group of rare and clinically heterogenous myeloproliferative neoplasms characterized by abnormal growth and activation of mast cells (MCs) and their precursors in the bone marrow and in various tissues and organs. Based on phenotype and extent of organ infiltration/dysfunction, a spectrum of disease variants can be recognized ranging from indolent SM (ISM) to aggressive SM (ASM) and mast cell leukemia (MCL). The fact that in all cases, including ISM who have a (near) normal life expectancy, neoplastic MCs display the same D816V KIT gene mutation points to additional mechanisms and molecular defects as responsible for ASM and MCL. So far, however, this issue has mainly been addressed with targeted resequencing studies of candidate gene panels. We thus decided to undertake an integrated molecular characterization study of ASM and MCL to identify novel, functionally relevant molecular lesions and/or clinically actionable signaling pathways.
Methods: A discovery panel including 6 patients with ASM and 6 patients with MCL was studied using whole exome sequencing (WES) and copy number variation (CNV) analysis. WES (80x) was performed on a Hiseq 2500 (Illumina). CNV was done using Cytoscan HD Arrays (Affymetrix). Paired normal/MC DNA was analyzed in all but 2 archival MCL cases for whom germline DNA was not available. A validation panel of 30 ISM, 5 smoldering SM and 20 additional ASM was also included in this study.
Results: In the discovery panel, WES identified a total of 1554 point mutations, small insertions and deletions. Seven hundred and eighty-five were non-silent mutations in 698 genes, with an average of 51 (range, 30-186) non-silent mutations per patient. Non-silent mutations included 354 missense mutations, 188 nonsense mutations, 145 frameshift insertions/deletions, 98 non-frameshift insertions/deletions. C to T transitions were by far the most frequent. Orthogonal validation estimated the accuracy of mutation calls at >95%. Interrogation of the COSMIC and OMIM databases revealed 42 known cancer genes. Among the missense mutations, 87 were predicted to have a high probability of being deleterious by Condel. MCL cases were found not to harbour a higher mutation load as compared to ASM cases. High resolution CN analysis showed that focal amplifications/deletions/loss-of-heterozygosity (LOH) were prevalent over arm-level alterations (found in 3 patients only).
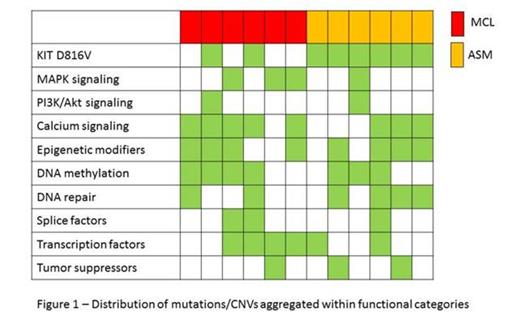
Genes were selected for further assessment when recurrently mutated in ≥2 patients or concurrently identified in WES and CNV analyses or previously associated with leukemogenesis or cancer pathogenesis. Among these, genes already reported to be affected by mutations in SM included TET2, NRAS, ASXL1, CBL, IDH1, SRSF2, SF3B1, RUNX1. We also identified genetic alterations in genes not previously implicated in SM pathogenesis including TP53BP1, RUNX3, NCOR2, CDC27, CCND3, EI24, MLL3, ARID1B, ARID3B, ARID4A, SETD1A, SETD1B, KDM1B, PRDM1, ATM, WRN. A long tail of infrequently mutated genes dominated, resulting in significant intertumoural heterogeneity. However, when genes were assigned to functional pathways to discern patterns of mutations across different patients, we found that PI3K/Akt and MAPK pathways, calcium pathway, chromatin modification, DNA methylation, and DNA damage repair were consistently affected (Figure 1).
Further assessment of the mutation frequency of selected genes within each pathway and functional validation at the protein level are currently ongoing in the validation panel. Preliminary findings on a tumor suppressor selected among those identified by WES show transcript and/or protein downmodulation due to inactivating mutations, transcriptional silencing or enhanced degradation in 17/20 ASM. Detailed results will be presented at the meeting.
Conclusions: WES and CNV analyses of ASM and MCL revealed a complex landscape, not unexpected when considering the clinical heterogeneity of these patients. Nonetheless, key pathways were found to be recurrently altered. Further investigation of selected candidate genes and pathways is warranted and will cast light on the cooperative genetic (and epigenetic?) events underlying the more aggressive forms of SM - paving the way to a better prognostic stratification and more effective treatment. <>This study was supported by ELN, AIL, AIRC, progetto Regione-Università 2010-12 (L. Bolondi), FP7 NGS-PTL project.
Soverini:Ariad: Consultancy; Bristol-Myers Squibb: Consultancy; Novartis: Consultancy. Valent:Novartis: Consultancy, Honoraria, Research Funding; Ariad: Honoraria, Research Funding; Bristol-Myers Squibb: Honoraria; Pfizer: Honoraria; Celgene: Honoraria. Cavo:Janssen-Cilag, Celgene, Amgen, BMS: Honoraria. Martinelli:Novartis: Consultancy, Speakers Bureau; BMS: Consultancy, Speakers Bureau; ROCHE: Consultancy; Pfizer: Consultancy; Ariad: Consultancy; AMGEN: Consultancy; MSD: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.