Abstract
SK and JJ: deserve equal credit; SS and JTP :senior authors
During early female embryogenesis, in each somatic cell, most of the genes on either the maternal or paternal X-chromosomes are randomly inactivated; this remains remarkably constant in the progeny of these cells throughout life. An X-inactive specific transcript gene (XIST), which encodes long non-coding RNA, is expressed from the inactive X-chromosome and plays a crucial role in this process. XIST covers the X-chromosome in cis and triggers genetic silencing, but its mechanism of action remains to be fully defined. XIST is both required and sufficient for inactivation of X-chromosomes and is continuously expressed throughout female life.
Clonal populations can be detected through indirect measures, such as the expression of surrogate genes as in the case of X-chromosome inactivation. X-chromosome inactivation has been used to define the clonality of malignant and premalignant disorders such as polycythemia vera (PV) and essential thrombocythemia (ET). We developed a quantitative, transcriptional clonality assay (qTCA) based on polymorphisms for five X-chromosome genes (MPP1, FHL1, IDS, BTK, and G6PD) (Swierczek S, Blood, 112;p.3168, 2008). We found that the allelic usage ratio of these genes varies among normal females and from tissue to tissue, but is the same in any given female in all blood cell lineages and is stable in time. It was previously reported that reactivation of inactivated X-chromosomes is seen in some human breast cancers (Richardson A., Cancer cell, 9; p.121, 2006,). However, these abnormalities did not lead to a global increase in X-chromosome transcription but were associated with overexpression of a small subset of X-chromosomal oncogenes and/or tumor suppressor genes (Thakur A., Mole Cancer Res, 2007). The conditional deletion of Xist inhematopoietic stem cells in female mice resulted in X-chromosome reactivation and led to complete penetrance of a highly aggressive PV/ET-like syndrome that progressed to invariably fatal lymphoma and/or acute leukemia (Yildirim E, Cell 152;p. 727, 2013).
In three PV/ET female patients, we observed expression of both alleles of the IDS and G6PD genes in clonal platelets and granulocytes, whereas in these three females only a single other X-chromosome allele was expressed. We concluded that reactivation of inactive IDS and G6PD, and perhaps other X-chromosome genes, occurred in the PV/ET clones of these females (Kim SJ & Swierczek S, ASH abstract #3225, 2014). We then investigated the epigenetic status of the IDS and G6PD genes in these patients.
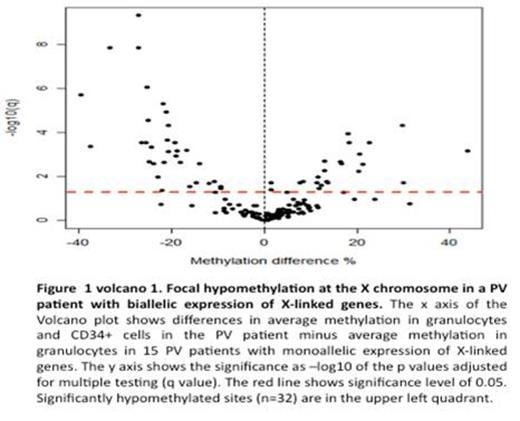
We performed chromatin immunoprecipitation of granulocyte DNA from all three patients using histone H3 lysine 27 trimethylation (H3K27me3) antibody to assess the level of polycomb silencing. The IDS and G6PD genes in these PV females had decreased the H3K27me3 compared to normal controls. We next performed DNA methylation analysis focused on the X-chromosome CpG sites located within 1kb from gene transcription start sites. We have analyzed 19 PV females 9 control females and 22 control males. We observed discrete regions showing focal hypomethylation in PV compared to control females (Fig. 1).
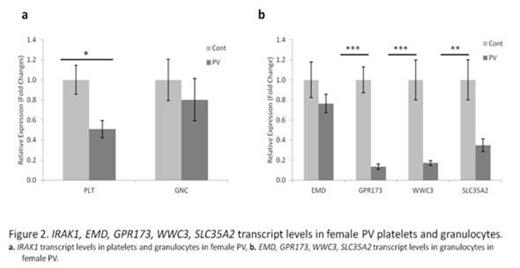
In the PV females, there were large regions of X-chromosome, which were composed of the same number of methylated and unmethylated regions as control females. However, there were also segments of X-chromosome genes, which were less methylated than in control females (Fig. 1). Some of these genes included variable hypomethylation in promoter regions. We then isolated clonal platelets and granulocytes, and transcripts of these genes were quantified by qT-PCR. Inexplicably, the transcripts of these genes were decreased in most PV females (p<0.05) (Fig. 2). The molecular basis of this unexpected decrease in transcripts of these hypomethylated X-chromosome genes in PV is currently being interrogated, including possible activation of anti-sense transcripts.
In conclusion, we demonstrate significant X-chromosome demethylation, which appears to correlate with reactivation of some X-chromosome genes in PV and ET females. Defining the molecular basis of these observations is important because the conditional reactivation of inactivated X-chromosome genes in hematopoietic stem cells leads to aggressive MPN-like disease in mice and expansion of their stem cells. Further, PV females are more likely to have PV and have a different clinical course.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal