Abstract
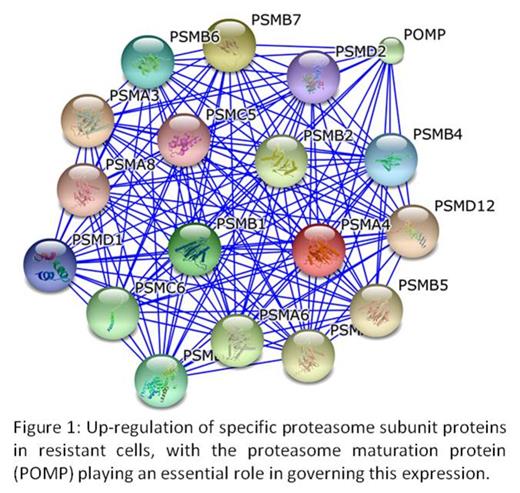
The range of new proteasome inhibitors currently in clinical or preclinical development hold tremendous promise for the treatment of haematological malignancies, in particular multiple myeloma, both in frontline and relapsed or refractory settings. However the development of resistance to proteasome inhibitors is a significant problem. To facilitate the analysis of this phenotype, a carfilzomib-resistant cell line was established and the effects on altered protein expression and ubiquitination levels determined. Label-free quantitative mass spectrometry was performed on whole cell extracts from the resistant model together with ubiquitin remnant motif antibody K-ε-GG enriched peptides for modification analysis. A significant number of proteins with altered expression and ubiquitination levels were positively identified. In particular, the proteasome maturation protein (POMP) was found to have a significantly up-regulated abundance level in the resistant phenotype, a protein essential for the assembly of standard proteasomes and immunoproteasomes (Figure 1). Abnormal ubiquitination levels were also detected on proliferating cell nuclear antigen (PCNA), significantly on Lysine-164 which is involved in DNA-repair pathways. Identifying the relevant biological pathways affected by proteasome inhibitors and the changes which mediate drug resistance may allow for optimal therapeutic use and potential new drug targets. POMP and PCNA may be important target molecules to help overcome the establishment of resistance to proteasome inhibitors in the treatment of multiple myeloma. Further work could examine broadening the therapeutic application of proteasome inhibitors that is currently confined to multiple myeloma and identifying the key proteins involved in common resistance mechanisms.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.