Abstract
Introduction: In recent years, the multiple myeloma (MM) treatment (Tx) landscape has evolved considerably, with the approval of effective novel agents, such as immunomodulatory drugs (thalidomide, lenalidomide, and most recently, pomalidomide) and proteasome inhibitors (bortezomib and carfilzomib; Chng et al, Leukemia, 2014). However, most patients relapse and patterns of relapse in MM can be heterogeneous (Alegre et al, Haematologica, 2002).
Tx rescue for MM relapse should begin at symptomatic clinical relapse (clinR) and probably earlier at asymptomatic biological relapse (BR; specifically when significant paraprotein relapse), according to the International Myeloma Workshop Consensus Panel (Rajkumar et al, Blood, 2011).
In the case of asymptomatic BR/progression, rescue Tx could be delayed in a subset of pts (Fernández de Larrea et al, Bone Marrow Transplant, 2014), particularly when M-component and hemoglobin levels are stable. Progressive increases of M-spike blood and/or urine could lead to rescue Tx, even in the absence of clinical symptoms, to avoid complications (ie, renal failure, plasmacytomas, bone lesions; Castelli et al, Clin Med Insights Oncol, 2013).
This Spanish registry (EPA-MMBR) is an observational prospective study to describe MM relapse patterns, comparing the impact of Tx decisions (starting Tx at BR vs delaying Tx until clinR). We present the updated preliminary results of this study.
Methods: MM pts in (or prior to) first or second BR who had achieved ≥ PR since their last Tx were included. Bi-monthly evaluations were performed. Forty-one Spanish sites are participating after approval from their independent ethics committees, with 410 pts expected to be included.
The main objective was to assess time to progression (TTP) from BR in both groups (pts who started Tx at the onset of asymptomatic BR vs pts who started Tx at clinR). Secondary objectives included demographic, clinical, and Tx characteristics; median time from BR to clinR; and response rates. Here we present the results of 138 pts with baseline data (48.4%) of a total of 285 registered.
Results: In the cohort evaluated (n = 138), mean age was 67.9 years, and 50.7% were male. MM types were IgG Κ (43.5%), IgG λ (22.1%), IgA Κ (16%), and IgA λ (7.6%). Prognostic stage at diagnosis according to the International Staging System (ISS) was II (32.6%) followed by I and III (22.7% each) and data not available (22%). Pts' cytogenetic risk included standard risk (20.8%), high risk (8.5%), and no cytogenetic alterations (32.3%).
More than half of pts (51.5%) had received autologous stem cell transplant, 13.2% consolidation and 21.1% maintenance. After first-line Tx, pts had achieved sCR (16.0%), CR (33.6%), VGPR (28.8%), or PR (21.6%). Pts with BR had a median time from diagnosis to BR of 2.66 years.
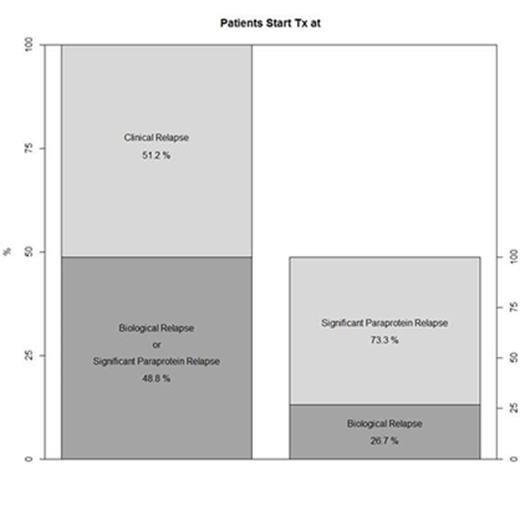
Tx was delayed until clinR in 51.2% of pts, and 48.8% had started Tx at BR (73.3% of whom had significant paraprotein relapse; Figure); there were no relevant differences in demographic and clinical characteristics between the 2 groups.
Median time to BR was 31.97 months. Median time from BR to clinR was 105.0 days. Among pts who started Tx at BR, most pts received lenalidomide-based Tx (75% at first relapse and 70.6% at second relapse).
At the time of the analysis, only 3 pts had disease progression (1 in each group).
In pts who started Tx at BR, first-line Tx after diagnosis was mainly bortezomib-based (73.3%). sCR was achieved in 20.0% of pts, CR in 33.3%, PR in 26.7%, and VGPR in 20.0%.
Since the beginning of the study, Tx was prescribed in 54 pts (39.1%). Since only 3 pts had progressed, further follow-up data, particularly in terms of TTP and survival, are needed to identify differences between these 2 strategies.
Conclusions: To our knowledge, this is the first prospective study in MM that evaluates the effects of starting Tx at BR vs starting Tx at clinR. In this updated cohort, we find that almost half of pts started Tx at BR (48.8%). We also found that a higher percentage of pts started Tx at BR with significant paraprotein relapse (73.3%), which seems to be a clear factor guiding Tx decision for the physician. Further follow-up and a complete study are needed to identify the differences between these 2 strategies and to define patients who could benefit from early Tx.
Percentage of pts who started Tx at clinR, BR, or significant paraprotein relapse (n = 138).
Percentage of pts who started Tx at clinR, BR, or significant paraprotein relapse (n = 138).
Gironella:Celgene Corporation: Consultancy, Honoraria. Fernández de Larrea:Celgene Corporation: Consultancy, Honoraria; Janssen: Consultancy, Honoraria. Lahuerta:Celgene Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees. Cabañas:Amgen: Membership on an entity's Board of Directors or advisory committees. Lostaunau:Celgene SLU: Employment. Vilanova:Celgene SLU: Employment. Alegre:Celgene Corporation: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.