Abstract
Background: Despite improvements in the treatment of relapsed/refractory multiple myeloma (RRMM), the disease remains incurable and new approaches to treatment are necessary. Clinical trials of vorinostat (V), a Class I/II pan-histone deacetylase (HDAC) inhibitor, in combination with proteasome inhibitors (PI) and immunomodulatory agents (IMiDs) have shown activity in RRMM. We report a Phase IIb, open-label, single institution study of V in combination with Ld in MM pts refractory to previous L-containing regimens.
Patients and Methods: Eligibility criteria included pts with RRMM who had received ≥1 prior anti-MM regimen, no prior HDAC inhibitor, and must have been refractory to L, defined as either progression of disease (PD) on L therapy or no clinical response (< minimal response (MR) to at least 2 cycles on a previous L-containing regimen. LVd was administered in a 28-day cycle with oral V 400mg d 1-7 and 15-21, L 25mg d 1-21 and dexamethasone 40mg d 1,8,15, and 22. The primary endpoint was overall response rate (ORR).
Results: 25 pts were enrolled from March 2012 to January 2014. Median age was 65 years (48-82), 18 (72%) were male. The median time from diagnosis to study entry was 4.95 yrs, median number of prior regimens was 5 (3-10); 24 (96%) had undergone at least 1 prior autologous transplant, 20 (80%) had prior PI exposure, and 9 (36%) had prior thalidomide. The last line of therapy prior to study entry was Ld in 16 (64%) and a non-L regimen in 9 (36%). Of pts on Ld prior to study entry, 11 were refractory and 5 relapsed/refractory to treatment. The last line of therapy in all 9 pts on a non-L regimen prior to study entry was a PI-containing therapy and in these pts the median time since last treatment with a L-containing regimen was 13.31 months (mos) (1.87-58.38).
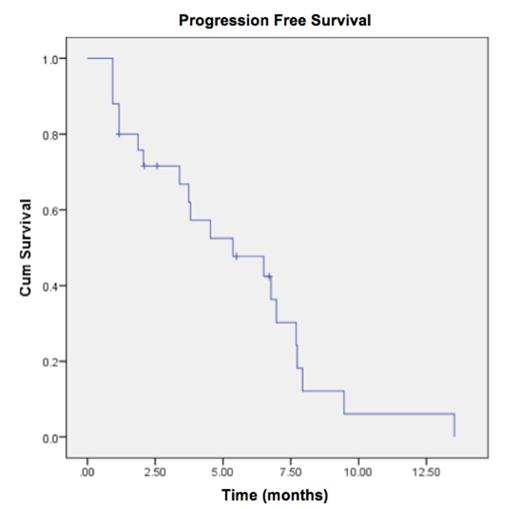
The ORR (≥ partial response (PR) was 24% (n=6); in addition 1 MR and 13 SD (52%) was observed for an overall objective response rate (≥ SD) of 80%. The median time to best response was 1.90 mos (0.93-3.67); median duration of response of pts in PR was 3.25 mos (95% CI 1.17-5.33). Five pts (20%) had PD after the first cycle of therapy. Of the 5 pts who were relapsed/refractory to Ld therapy prior to study entry, 1 achieved PR, 1 MR, and 3 SD. The median PFS of the entire cohort was 5.4 mos (95% CI 1.58-9.15), with 6-month (mo) PFS rate 48% and 12-mo PFS rate 6% (Figure 1). There was no significant difference in ORR between pts whose last line of therapy prior to study entry was Ld vs. a non-L regimen: ORR 19% and 33%, respectively (p=0.42). Median PFS was 3.80 mos (95% CI 3.16-4.44) vs. 6.97 mos (95% CI 3.06-10.87) in pts whose last line of therapy prior to study entry was Ld vs. a non-L regimen, respectively (p = 0.084, log rank test).
All 25 pts (100%) had at least 1 drug-related adverse event (AE). Common AEs (any grade, most of which were Grade 1/2) at least possibly attributable to the study drugs included diarrhea (72%), fatigue (72%), anorexia (28%), nausea (28%), dysgeusia (28%). Most grade 3/4 AEs were related to hematologic toxicities with Grade 3/4 anemia, neutropenia, and thrombocytopenia occurring in 20%, 48%, and 32%, respectively. Dose modifications for either V or L were necessary in 18 pts (72%) due to thrombocytopenia, neutropenia, and/or diarrhea. 8 pts (32%) experienced at least one serious AE, of which only 2 were considered possibly related to therapy.
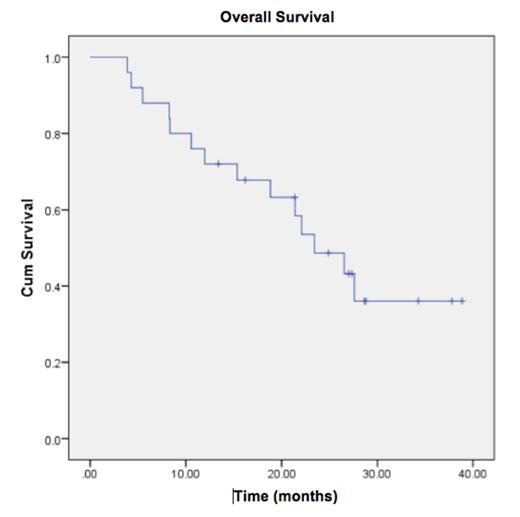
Only one patient was withdrawn from study due to toxicities (cytopenias), other reasons for study discontinuation included PD 20 pts (80%) and consent withdrawal in 3 (12%). Median OS was 23.4 mos (95% CI 16.42-30.45), with 12-mo and 24-mo OS 72% and 49%, respectively (Figure 2).
Conclusions: The combination of V with Ld resulted in ORR 24% and overall objective response rate of 80%, an appreciable clinical benefit considering the study group was a heavily pre-treated population with RRMM. Notably, in pts progressing on Ld immediately prior to study entry, the addition of V to Ld appears to enhance the anti-myeloma effect of L and re-sensitize pts to Ld therapy. Further studies are warranted to better characterize the role of vorinostat and other novel HDAC inhibitors in combination with IMiDs and PIs in RR MM.
McBride:Celgene: Speakers Bureau. Bilotti:Pharmacyclics LLC, an AbbVie Company: Employment. Vesole:Idera Pharmaceuticals: Research Funding; Celgene Corporation: Speakers Bureau. Biran:Celgene: Speakers Bureau. Siegel:Celgene Corporation: Consultancy, Speakers Bureau; Amgen: Speakers Bureau; Takeda: Speakers Bureau; Novartis: Speakers Bureau; Merck: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.