Abstract
Invariant NKT (iNKT) cells are glycolipid-reactive alpha/beta T cells which have an important role in the regulation of GVHD after allogeneic bone marrow transplantation. During thymic development, murine iNKT cells divide into three transcriptionally distinct lineages-NKT1, NKT2, and NKT17 that differ in their cytokine expression profile both at rest and upon antigen recognition via their TCR. Given that the lineage profile of iNKT cells varies dramatically between inbred strains of mice, it has been postulated that recognition of allospecific glycolipids determines iNKT cell lineage-fate decisions. Therefore, we challenged this hypothesis in a murine model of prenatal allogeneic transplantation to determine if the lineage commitment of immature iNKT cells was intrinsically programmed or extrinsically regulated by the allospecific environment during development.
Prenatal allogeneic chimeras were established by in utero transplantation of E14 fetal liver light density cells into age-matched allogeneic fetal recipients (Balb/c to B6 or B6 to Balb/c). In this model, immature iNKT cells of both donor and host origin have the capacity to participate in education as CD1d on bone marrow-derived cells regulate the maturation of developing iNKT cells. This permitted an analysis of the impact of either host-to-donor or donor-to-host environmental cues in directing iNKT cell lineage-fate decisions. iNKT cell populations were identified using flow cytometric analysis of the transcription factors PLZF and T-bet. The lineage profile for donor and host thymic iNKT cells from chimeric mice were compared to the thymic iNKT cell population in naïve controls.
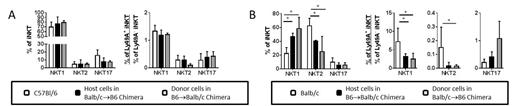
As shown, B6 iNKT cells in prenatal chimeras exhibited a predominance of the NKT1 lineage in either the donor or the host situation similar to their frequency in naïve B6 controls (figure A). Conversely, Balb/c iNKT cells in both the donor and the host situation exhibited skewing toward an NKT1 lineage profile and away from the NKT2 lineage bias seen in naïve Balb/c controls (figure B). Furthermore, the expression of the H-2d MHC class I-reactive Ly49A receptor by Balb/c iNKT cells strongly correlates with the NKT1 lineage fate in control animals. However, both donor and host Balb/c cells demonstrated reduced correlation between Ly49A expression and NKT1 lineage fate indicating that the presence of H-2b expressing B6 cells diminished the ability of H-2d -reactive Ly49A to dictate lineage fate decisions.
This study uniquely demonstrates the potential for cell-extrinsic signals in guiding iNKT cell lineage fate in an asymmetric fashion. Specifically, we find that: 1) the B6 iNKT lineage profile is intrinsically determined and unaffected by exposure to allogeneic Balb/c cells during development; 2) the Balb/c iNKT cell lineage profile is extrinsically determined and dominantly skewed toward an NKT1 lineage by exposure to even small numbers of B6 cells during development; and 3) the exposure to B6 cells overrides the contribution of Ly49A to developmental decisions made by Balb/c iNKT cells. Future studies will explore the regulatory interactions that govern allospecific iNKT cell lineage fate decisions and the resulting impact on the pro-inflammatory or immunoregulatory function of iNKT cells in clinically-relevant models.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.