Abstract
Allogeneic hematopoietic stem cell transplantation (HCT) is a potentially curative treatment for malignant and non-malignant diseases. The late complications in the myeloablative setting, including secondary malignancies (SM), have been reported in several studies. For example, the CIBMTR reported a 10-years cumulative incidence (CI) of 1%. The recent development of reduced intensity conditioning (RIC) for older patients has dramatically increased the number of HCT. Because they are typically performed in older patients, the analysis of post-transplant SM in this population is of particular interest. However, reported data are scarce in this specific population in the RIC setting. As a consequence, we performed a single center retrospective study to assess the risk of SM with extended follow-up in patients transplanted after a RIC regimen.
Patients transplanted at our center for a hematological malignancy after a RIC regimen between 01/01/2000 and 12/31/2012 were screened. The analysis was performed in the subgroup of patients alive and disease-free 2 years after transplant. Probabilities of overall survival (OS) were calculated from using the Kaplan-Meier estimate. Non-relapse mortality (NRM) included all causes of death without prior relapse, occurring at any time after transplant. All probabilities were calculated from the date of transplantation. CI curves were used for NRM and SM in a competing risk setting with relapse as a competing event for NRM and death for SM. The analysis of risk factors for SM was performed using the Fine and Gray model.
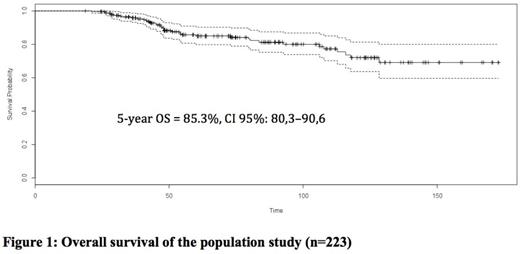
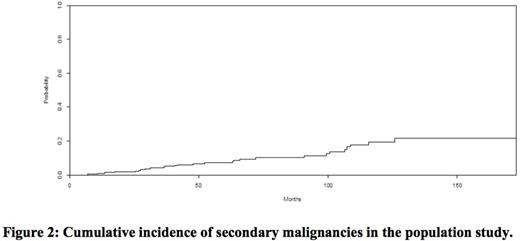
Five hundred and six patients were transplanted after a RIC regimen during the period of study. Among them, 223 were alive and disease-free at 2 years and thus included in the study. The median follow up was 73 months (24.3; 173). The median age was 55 years (18; 67). The characteristics of patients, diseases and transplants are summarized in Table 1. The 5-year OS was 85.3 % (CI 95%: 80.3 - 90.6) (Figure1). The 5-year OS in the no SM vs SM groups were 85% vs 83.5% while the 10-year OS in the same groups were 79.4% vs 33.3%, respectively, (p=0.0052). Thirty-eight patients died: 11 of relapse, 7 of SM, 15 of chronic GVHD or infection, 2 of vascular complications, 2 of unknown causes and one of suicide. Twenty-five patients developed a SM (excluding non-melanoma skin cancer) at a median time of 63.7 months (13.57; 172) after transplant. The 5 and 10-year CI of SM were 6.4%±1.7% and 18,7%±3.9%, respectively (Figure 2). The SM were distributed as follow: lung cancer (n=5), squamous cell carcinoma (1 throat, 1 tongue and 1 sinus), oesophageal cancer (n=3), colon cancer (n=2), Hodgkin lymphoma (n=2), bladder cancer (n=2), prostate cancer (n=2), melanoma (n=1), ovarian cancer (n=1), endometrial cancer (n=1), sarcoma (n=1), glioblastoma (n=1) and an unknown primary cancer (n=1). Multivariate analysis identified CMV seropositivity of donor and/or recipient (HR 4,4, p=0,016), non-sibling donor (HR 2,7, p=0,01) and a female donor for a male recipient (HR 2,7, p=0,012) as risk factors for SM.
In conclusion, we report a high incidence of SM as a late complication of HCT in patients with a median age of 55 years after an extended follow-up. This result strongly suggest that older patients should be carefully followed for a prolonged period after HCT and that attention must be paid to usual recommended screening tests and classical risk factors like tobacco and alcohol.
Characteristics of patients, diseases and transplants (n=223)
| . | Value . |
|---|---|
| Median age (years) (range) | 55 (18; 67) |
| Gender | |
| Male Female | 133 (60%) 90 (40%) |
| Diseases | |
| AML ALL MDS CML MPD NHL HL CLL MM SAA | 89 (40%) 12 (5%) 18 (8%) 2 (1%) 2 (1%) 38 (17%) 9 (4%) 11 (5%) 29 (13%) 13 (6%) |
| Status at transplant | |
| Early stage Advance stage | 106 (48%) 117 (52%) |
| Prior autologous transplantation | 85 (38%) |
| Conditioning regimen | |
| Fluda + Bu 2 days TBI 2Gy + Fluda Fluda + Cy + TBI 2 Gy Cy + TBI 2Gy Other | 133 (60%) 33 (15%) 24 (11%) 12 (5%) 19 (9%) |
| Source of stem cell | |
| BM PBSC CB | 20 (9%) 179 (80%) 24 (11%) |
| Rabbit ATG | |
| ATG No ATG | 156 (70%) 67 (30%) |
| Donor | |
| HLA-identical sibling Matched related donor Matched unrelated donor Cord blood | 105 (47%) 69 (31%) 25 (11%) 24 (11%) |
| Prophylaxis of GVHD | |
| CsA CsA + MTX CsA + MMF | 70 (31%) 82 (37%) 71 (32%) |
| Period of transplant | |
| 2000-06 2007-12 | 87 (39%) 136 (61%) |
| . | Value . |
|---|---|
| Median age (years) (range) | 55 (18; 67) |
| Gender | |
| Male Female | 133 (60%) 90 (40%) |
| Diseases | |
| AML ALL MDS CML MPD NHL HL CLL MM SAA | 89 (40%) 12 (5%) 18 (8%) 2 (1%) 2 (1%) 38 (17%) 9 (4%) 11 (5%) 29 (13%) 13 (6%) |
| Status at transplant | |
| Early stage Advance stage | 106 (48%) 117 (52%) |
| Prior autologous transplantation | 85 (38%) |
| Conditioning regimen | |
| Fluda + Bu 2 days TBI 2Gy + Fluda Fluda + Cy + TBI 2 Gy Cy + TBI 2Gy Other | 133 (60%) 33 (15%) 24 (11%) 12 (5%) 19 (9%) |
| Source of stem cell | |
| BM PBSC CB | 20 (9%) 179 (80%) 24 (11%) |
| Rabbit ATG | |
| ATG No ATG | 156 (70%) 67 (30%) |
| Donor | |
| HLA-identical sibling Matched related donor Matched unrelated donor Cord blood | 105 (47%) 69 (31%) 25 (11%) 24 (11%) |
| Prophylaxis of GVHD | |
| CsA CsA + MTX CsA + MMF | 70 (31%) 82 (37%) 71 (32%) |
| Period of transplant | |
| 2000-06 2007-12 | 87 (39%) 136 (61%) |
Milpied:Celgene: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract