Abstract
Introduction: Aberrant DNA methylation is frequently found in hematologic malignancies where it is associated with altered gene expression. DNA hypomethylating agents (DNMTi), e.g. 5-aza-2'-deoxycytidine (DAC), are used for both global and gene-specific in vivo demethylation and offer a therapeutic option in myelodysplastic syndromes (MDS) and AML. DNMTi have already been utilized to upregulate suppressed fetal hemoglobin (HbF) in adult patients (pts) suffering from hemoglobinopathies. Here we systematically investigated the potential of DAC for in vitro induction of erythroid differentiation as well as HbF expression in the bipotent myeloid leukemia cell line K562 and in vivo in a clinical treatment situation in MDS pts.
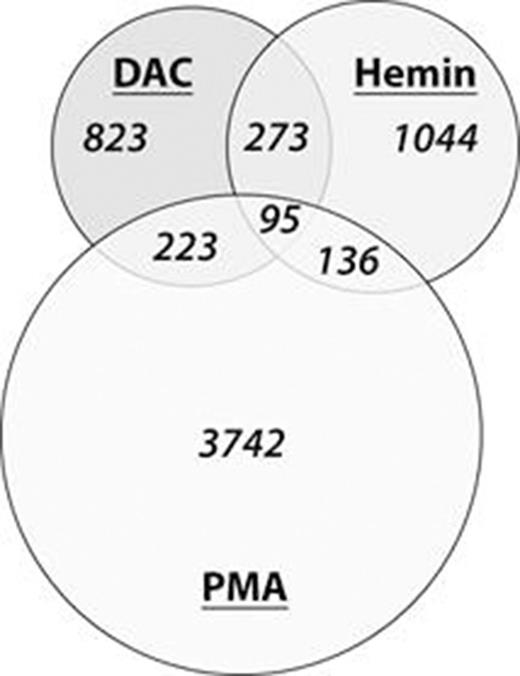
Methods and Results: We treated K562 cells with non-toxic concentrations of DAC (100 nM, three 24 hour pulses), hemin (50 nM) and phorbol myristate acetate (PMA, 5 nM). DAC treatment led to morphological changes indicating erythroid but not megakaryocytic differentiation. This was confirmed by benzidine staining where DAC (13% positive cells) and hemin (58%) but not PMA treated cells (0%) became positive for hemoglobin synthesis. Lack of CD41 detection by FACS analysis for DAC and hemin indicated absence of megakaryocytic differentiation. Transcriptome profiling by mRNA expression arrays (Affymetrix GeneChip® HG U133 Plus 2.0) revealed highest similarity between hemin and DAC treatment by unsupervised hierarchical clustering, followed by vehicle control and untreated cells. The transcriptome of PMA treated cells clustered most distantly to all other treatments. Both, DAC and hemin induced moderately balanced up- and downregulation of transcripts to an almost identical extent. 1414 transcripts were >2 fold upregulated and 1505 were >2 fold downregulated upon DAC treatment, whereas 1548 were up- and 2404 were downregulated in hemin treated cells, respectively. The extent of transcriptome dynamics was considerably stronger upon PMA treatment, where 4196 and 3780 transcripts were up- and downregulated, respectively. When intersecting transcriptome changes between the 3 drug treatments (Fig. 1), 368 out of 1548 (23.7%) upregulated transcripts in hemin treated cells were concordantly upregulated upon DAC treatment. The overlap of upregulated transcript was lower compared to PMA treated cells (14.9%). GO analyses of upregulated transcripts identified terms related to erythropoesis and iron metabolism among the top regulated groups of transcripts in DAC treated cells whereas terms related to megakaryocytic differentiation did not show significance. Particularly strong differences of transcripts were observed for a1-, a2-, Ag-, e- and z-globin expression upon DAC and hemin treatment, whereas b- and d-globin were expressed at low levels. These changes were not observed for PMA treated cells. Induction of a- and Ag-globin on mRNA level resulted in enrichment of a- and Ag-globin protein to 15.8% of total cellular protein amount, and consequently in HbF formation in K562 cells as assessed by reversed phase and anion exchange chromatography. HbF levels in peripheral blood were measured from 16 MDS pts, median age 74 years (range 66-78) also treated with a 3-day DAC schedule. Median HbF fraction at baseline was 0.4% (0.1-3.9%) of total hemoglobin with 6 pts (37.5%) exhibiting increased HbF levels (>1%) already before treatment. In 13/16 (81%) pts, increase of HbF with a median increment of 1.2% (range 0.3-3.7%) was observed. In 3 pts, HbF decreased over the treatment course. Median number of courses until maximum increment was 3 (2-6). HbF levels in 2 pts with AML and 1 with pancreatic cancer treated with nucleoside analogues without demethylating activity (cytosine arabinoside and gemcitabine, respectively) according to standard chemotherapy protocols served as control group and did not show comparable increments.
Conclusions: We describe an erythroid differentiation program, from transcriptome level to HbF protein formation, induced by the hypomethylating agent DAC in the bipotent cell line K562. This DAC-mediated differentiation process is specific for erythropoesis but not megakaryopoesis. This is substantiated by in vivo upregulation of HbF upon DAC adminstration in MDS pts. Therefore, we propose to utilize HbF expression as potential biomarker during DAC treatment.
Intersection of >2 fold upregulated transcripts in K562 cells upon drug treatment.
Intersection of >2 fold upregulated transcripts in K562 cells upon drug treatment.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.