Abstract
Background: Unlike acute lymphoblastic leukemia, data on acute myelogenous leukemia (AML) in the adolescents and young adults (AYA) are limited and generally derived from clinical trials, which are mostly conducted in academic centers. It is unclear whether the choice of treatment facility influences clinical outcome independent of disease biology and socio-demographic factors. This study used the National Cancer Data Base, a nationwide oncology database covering 70% of the US cancer population, to address this question.
Methods: We identified 11,712 AYA patients who were diagnosed with AML from 2003-2011. We excluded patients who were: a) 15-17 years of age (n = 3,104) as treatment facility data were not available for age <18 years; b) not treated at the reporting facilities (n = 415) as follow-up might be incomplete; c) treated at treatment facilities with ≤100 new cancer cases/year (n = 18); and d) those without follow-up data (n = 47). Treatment facilities were classified as academic, comprehensive community (>500 new cancer cases/year), or community (101-500 new cancer cases/year) cancer programs. AMLs harboring t(15;17), inv(16), or t(8;21) were defined as good-risk, while the rest were considered non-good risk. Overall survival from the time of AML diagnosis was the primary outcome. We used multivariate imputation by chained equation to impute missing values, weighted Cox regression to obtain average hazard ratios (HRs), and pooled Wald c2 statistics to detect interaction.
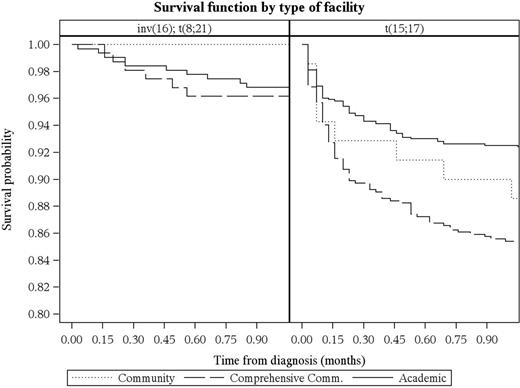
Results: We included 8,128 AYA patients with AML in the study and 25.2% were good risk. Among the good risk AMLs, 11.6%, 12.3%, and 76.1% had t(8;21), inv16, and t(15;17), respectively. The distribution of patients by treatment facility were academic (64.1%), comprehensive community (32.9%), and community (3.0%). After detecting an interaction between AML risk and type of treatment facility (P=0.038), we stratified treatment facility by AML risk and found significant differences in overall survival among good risk (P=0.02) but not non-good risk AML (P=0.19). This survival difference persisted even after adjusting for demographic (age, sex, race/ethnicity), socioeconomic (income, education, medical insurance), geographic (area of residence, travel distance), co-morbidities (Charlson-Deyo score), and treatment (receipt of chemotherapy) factors. Patients with good risk AML treated in comprehensive community cancer programs had a higher risk of death compared to those treated in academic programs (adjusted HR: 1.41, 95% CI: 1.11-1.79). For community cancer programs, the risk of death was also higher but did not reach statistical significance (adjusted HR: 1.22, 95% CI: 0.68-2.21). ). Subset analysis showed that the survival difference among those with good risk AML was observed in t(15;17) or acute promyelocytic leukemia (APL; P=0.02) but not in t(8;21) or inv16 (P=0.79). The disparity occurred primarily during the first month of APL diagnosis, wherein the risk of death was two-fold higher in community programs (Figure).
Conclusions: Among AYA patients with AML, treatment at academic cancer programs was associated with a better survival, but only for the APL subgroup. Because APL comprises a fifth of all AMLs in the AYA and is a highly curable cancer, health care delivery research in APL should become a priority in this age group.
Al-Kali:Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.