Abstract
Background
The incorporation of novel agents (NA) for multiple myeloma (MM) has improved the response rates (RR), overall survival (OS), and progression free survival (PFS) when compared to conventional agents (CA). Unfortunately, relapse is inevitable and few studies focus on patterns of relapse, especially in non-transplant patients (pts). We aim to describe the different patterns of relapse in non-transplant MM pts and determine if any pre-treatment clinical or disease characteristics can predict the patterns relapse. We will evaluate whether NA treated pts have higher rates of aggressive relapse with plasmacytomas or plasma cell leukemia (Leuk Res. 2009 Aug;33(8):1137-40). Secondly, RR and PFS for pts treated with CA vs NA will be described.
Methods
A retrospective evaluation of 156 consecutive newly diagnosed non-transplant eligible MM pts at Princess Margaret Cancer Centre receiving at least two consecutive cycles of CA or NA from 1999 to 2015. CA included steroids and alkylators while NA had immunomodulatory (IMiD) drugs (thalidomide, lenalidomide) and proteasome inhibitors (PI) (bortezomib). Response type was defined by the revised International Myeloma Working Group criteria (Leukemia. 2006 Sep;20(9):1467-73. Epub 2006 Jul 20); relapse patterns as defined in the Spanish Registry (Haematologica. 2002 Jun;87(6):609-14)
Results
For 156 non-transplant MM pts: 81 (52%) male, average age 76 yrs, 87 (56%) treated with NA (thalidomide=15; PI=52). Baseline characteristics were not significantly different between groups (Table 1). Sixty three (52%) pts had a clinical relapse, 37 (30%) pts had a biochemical relapse, and 22 (18%) were switched immediately to second line therapy given suboptimal response (lack of clinical benefit or PD). Six pts relapsed with isolated plasmacytomas (4 CA vs 2 NA). There was one case of plasma cell leukemia relapse in an IMiD-treated pt.Twenty seven (17.3%) pts had not relapsed at the time of analysis and had ongoing follow-up. There was no significant difference in the types of relapse patterns for pts treated with CA versus NA (p=0.26) or for CA versus IMiD versus PI therapy (p=0.22). Pts with insufficient response to first line chemotherapy were more likely to have a 17p deletion (p=0.07). All pts with a biochemical relapse did not have a 17p deletion. The median follow-up time was 16.4 (range 0.6 to 99) months (mo) for CA vs. 19.6 (range 0.4 to 107) mo for NA.
Patient Characteristics
| Relapse Pattern - Mean (sd) . | ||||
|---|---|---|---|---|
| . | Clinical n =63 . | Biochemical n =37 . | Insufficient n=22 . | p -value . |
| Hgb | 108 (17) | 99 (21) | 110 (18) | 0.08 |
| WBC | 6.1 (2.4) | 6.4 (3.3) | 5.6 (1.9) | 0.72 |
| Plt | 231 (107) | 224 (117) | 237 (103) | 0.64 |
| Ca | 2.5 (0.3) | 2.5 (0.4) | 2.4 (0.3) | 0.58 |
| Cr | 125 (92) | 130 (87) | 136 (133) | 0.92 |
| B2M | 492 (537) | 596 (448) | 618 (618) | 0.19 |
| Alb | 37 (7) | 36 (6) | 36 (5) | 0.29 |
| CRP | 6.7 (7.4) | 9.3 (19.1) | 13.0 (17.5) | 0.56 |
| Relapse Pattern - Count (%) | ||||
| Conventional Novel | 35 (56) 28 (44) | 15 (41) 22 (59) | 13 (59) 9 (41) | 0.26 |
| IgG IgA FLC Other | 34 (54) 17 (27) 10 (16) 2 (3) | 23 (62) 6 (16) 8 (22) 0 (0) | 14 (63) 4 (18) 4 (18) 0 (0) | 0.78 |
| Kappa Lambda | 32 (58) 23 (42) | 19 (59) 13 (41) | 12 (57) 9 (43) | 0.99 |
| Chr 13 Del | 8/29 (28) | 7/10 (41) | 3/9 (33) | 0.64 |
| t(4,14) | 2/29 (7) | 3/15 (20) | 0/8 (0) | 0.34 |
| 17p Del | 4/28 (14) | 0/15 (0) | 3/9 (33) | 0.07 |
| Extramed. Inv. | 4 (6) | 1 (3) | 1 (5) | 0.85 |
| Relapse Pattern - Mean (sd) . | ||||
|---|---|---|---|---|
| . | Clinical n =63 . | Biochemical n =37 . | Insufficient n=22 . | p -value . |
| Hgb | 108 (17) | 99 (21) | 110 (18) | 0.08 |
| WBC | 6.1 (2.4) | 6.4 (3.3) | 5.6 (1.9) | 0.72 |
| Plt | 231 (107) | 224 (117) | 237 (103) | 0.64 |
| Ca | 2.5 (0.3) | 2.5 (0.4) | 2.4 (0.3) | 0.58 |
| Cr | 125 (92) | 130 (87) | 136 (133) | 0.92 |
| B2M | 492 (537) | 596 (448) | 618 (618) | 0.19 |
| Alb | 37 (7) | 36 (6) | 36 (5) | 0.29 |
| CRP | 6.7 (7.4) | 9.3 (19.1) | 13.0 (17.5) | 0.56 |
| Relapse Pattern - Count (%) | ||||
| Conventional Novel | 35 (56) 28 (44) | 15 (41) 22 (59) | 13 (59) 9 (41) | 0.26 |
| IgG IgA FLC Other | 34 (54) 17 (27) 10 (16) 2 (3) | 23 (62) 6 (16) 8 (22) 0 (0) | 14 (63) 4 (18) 4 (18) 0 (0) | 0.78 |
| Kappa Lambda | 32 (58) 23 (42) | 19 (59) 13 (41) | 12 (57) 9 (43) | 0.99 |
| Chr 13 Del | 8/29 (28) | 7/10 (41) | 3/9 (33) | 0.64 |
| t(4,14) | 2/29 (7) | 3/15 (20) | 0/8 (0) | 0.34 |
| 17p Del | 4/28 (14) | 0/15 (0) | 3/9 (33) | 0.07 |
| Extramed. Inv. | 4 (6) | 1 (3) | 1 (5) | 0.85 |
Sixty (38%) pts achieved VGPR/CR/sCR, 53 (34%) PR, 35 (22%) SD, and 8 (5%) PD with upfront therapy. VGPR/CR/sCR was seen in 13 (21%) pts with CA vs 47 (78%) with NA (p<0.01). For NA, 28 (47%) pts in the PI-based group achieved VGPR/CR/sCR compared to 19 (32%) in IMiD-based (p<0.01).
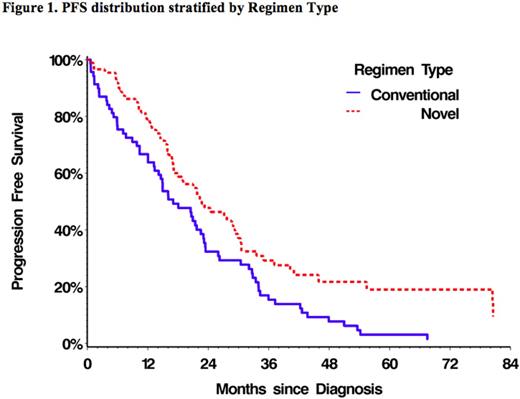
The median PFS for all pts was 21 (95% CI 17-23) mo, with 17 (95% CI 13-23) mo for CA vs 23 (95% CI 17-29) mo in NA. There is a statistically significant difference between CA and NA in PFS (p=0.0045; Figure 1).
Discussion
In non-transplant MM pts, we did not find a significant difference in the patterns of disease relapse between those treated with CA versus NA. Baseline characteristics such as renal failure or type of treatment do not seem to predict for the pattern of relapse; except the presence of 17p deletion trended toward more treatment failure. We note that the number of pts with aggressive relapses (plasmacytomas or plasma cell leukemia) was low and this limits our ability to detect differences in outcomes and baseline factors. Pts treated with NA continue to have better RR and PFS than those treated with CA. Future work with longer follow-up intervals is needed in order to capture late relapses, better describe relapse patterns with NA as well as understanding disease biology.
Chen:Celgene: Consultancy, Honoraria, Research Funding. Prica:Janssen: Honoraria; Celgene: Honoraria. Reece:Lundbeck: Honoraria; Janssen-Cilag: Consultancy, Honoraria, Research Funding; Merck: Research Funding; Millennium Takeda: Research Funding; Bristol-Myers Squibb: Research Funding; Otsuka: Research Funding; Novartis: Honoraria, Research Funding; Onyx: Consultancy; Amgen: Honoraria; Celgene: Consultancy, Honoraria, Research Funding. Tiedemann:Janssen Ortho: Honoraria; Celgene: Honoraria; Amgen: Honoraria. Kukreti:Celgene: Honoraria; Amgen: Honoraria; Lundbeck: Honoraria; Roche: Honoraria; Janssen Ortho: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.