Abstract
Background: In our previous work (56th ASH poster, No.2416), we developed a novel cell transplantation system named MagIC-TT. The purpose of this study is to explore whether the MagIC-TT can promote hematopoietic recovery in the mice experiment and illustrate it¡¯s mechanism both in vivo and in vitro.
Methods: 1) In vivo study: With regard to auto-transplantation, the C57BL/6 CD45-GFP cells were sorted and magnetized from the bone marrow of C57BL/6-Tg(CAG-EGFP) mice. Forty C57BL/6 female mice (2 groups, twenty mice each group) were transplanted into the femur cavity with or without magnetic field (M or W group), after 7.5Gy irradiation. Following transplantation, the survival of mice, hematopoiesis as well as GFP+ cells in different tissues, such as peripheral blood, bone marrow, liver, spleen, thymus and lung etc. were observed. Femurs of recipients were decalcified with our own derived semi-solid decalcification (SSD) technique to illustrate the distribution, proliferation of donor cells and the relationship between recipients and donor cells.
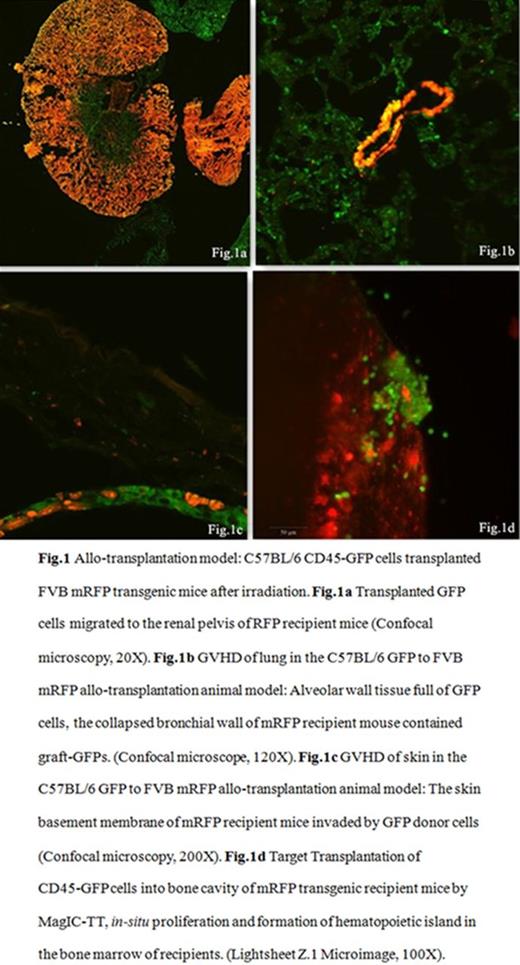
Allo-transplantation: The C57BL/6 CD45-GFP cells were injected into the femur cavity of FVB mRFP transgenic mice (sponsored by Prof. XH Wu, Fudan University, Shanghai, China) after 7.5Gy irradiation. GVHD was observed in addition to what was done in auto-transplantation.
2) In vitro study: Magnetized CD45-GFP cells and non-magnetized BMSC-RFPs were cultured respectively or co-cultured with or without magnetic field (M or W group). The magnetic field was added to the top or the bottom of cell culture dish. Cell morphology, cell proliferation, cell viability, as well as cell migration, transwell migration and matrigel migration assays induced by magnetism were studied. The interaction of CD45-GFP cells and BMSC-RFPs was observed by confocal microscope, electronic microscope, immunohistochemical staining, western blot, real-time PCR and deep sequencing.
Results: 1) In vivo study: During the first few hours after transplantation, lots of magnetized CD45-GFP cells resided within the femur and knee joints in M group while few in W group. Many GFP cells migrated into the lung soon after transplantation in the W group (P =0.046), followed by other organs such as kidney and skin (Fig.1). FACS showed that more GFP+ cells resided within the target femurs than the controls (Table.1). With SSD, frozen sections, confocal microscope and Lightsheet Z.1 Microimage (Carl Zeiss); transplanted GFP+ cells and their micro-environment were all well demonstrated (Fig.1). On removal of magnetic field, CD45-GFP cells were observed to migrate into the spleen, kidney, gut and other organs, showing the slow release of target transplanted cells from femur. GVHD on skin and lung etc. were observed in C57BL/6 to FVB allogenic transplanted mice (Fig. 1). The hematopoietic recovery in M group occurs much earlier than the controls, especially for the platelets, 10.67d ¡À 1.53d vs 14.75d ¡À 2.06d (M vs W group, P =0.035).
2) In vitro study: With the help of MagIC-TT, CD45-GFP cells can migrate through the matrigel and transwell membranes much more efficiently. The magnetized CD45-GFP cells advance toward the inner roof of petri dish in the culture medium, and attach to BMSC-RFP growing on the inner roof of dish and proliferate in the niche composed by BMSC-RFP under the effect of magnetic field (Fig.2).
Conclusion: MagIC-TT could enhance CD45+ cells target migration, improve stem cell homing and proliferation efficiency, as well as promotion hematopoietic recovery in vivo. This study would shed light on current Hematological Stem Cell Transplantation (HSCT) and other cell therapies.
The FACS results of femurs of CD45-GFP cells injected into C57 mice, at 0.5h, 24h and 72h respectively.
| group . | 0.5h£¨%£© . | p . | 24h£¨%£© . | p . | 72h£¨%£© . | p . | |||
|---|---|---|---|---|---|---|---|---|---|
| *LC . | **RT . | *LC . | **RT . | *LC . | **RT . | ||||
| BMM | 0.017¡À0.006 | 0.497¡À0.151 | 0.040 | 0.080¡À0.026 | 1.573¡À0.508 | 0.030 | 0.190¡À0.139 | 1.960¡À0.809 | 0.049 |
| BMW | 0.017¡À0.012 | 0.050¡À0.017 | 0.184 | 0.013¡À0.006 | 0.027¡À0.015 | 0.184 | 0.023¡À0.015 | 0.320¡À0.434 | 0.368 |
| P | 1.000 | 0.007 | 0.013 | 0.006 | 0.108 | 0.036 | |||
| group . | 0.5h£¨%£© . | p . | 24h£¨%£© . | p . | 72h£¨%£© . | p . | |||
|---|---|---|---|---|---|---|---|---|---|
| *LC . | **RT . | *LC . | **RT . | *LC . | **RT . | ||||
| BMM | 0.017¡À0.006 | 0.497¡À0.151 | 0.040 | 0.080¡À0.026 | 1.573¡À0.508 | 0.030 | 0.190¡À0.139 | 1.960¡À0.809 | 0.049 |
| BMW | 0.017¡À0.012 | 0.050¡À0.017 | 0.184 | 0.013¡À0.006 | 0.027¡À0.015 | 0.184 | 0.023¡À0.015 | 0.320¡À0.434 | 0.368 |
| P | 1.000 | 0.007 | 0.013 | 0.006 | 0.108 | 0.036 | |||
*LC: Control femur without magnetic field (W group); **RT: Treated femur with magnetic field (M group).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.