Abstract
Introduction.
Invasive fungal infections (IFI) represent a major limiting factor for the successful outcome of hematologic patients undergoing allogeneic hematopoietic stem cell transplantation (HSCT). Consequently, the identification of pre-transplant clinical risk factors for IFI may be considered a tool of paramount importance leading to targeted prophylactic measures and improved treatment strategies. Aim of the present study was to evaluate the pre-transplant hematopoietic cell transplantation co-morbidity index (HCT-CI) risk score and its impact on the outcome of HSCT recipients who develop an IFI.
Methods.
Between January 2009 and March 2015, 301 consecutive patients with complete HCT-CI risk score assessment, who received a first allogeneic HSCT in a single Stem Cell Transplantation Center, were analyzed. Overall, 62% of the patients had an acute leukemia and donor source was an alternative donor in 67% of the cases; 58% of the patients had a low or intermediate HCT-CI risk score (0-2) whereas 42% of the patients had a high HCT-CI score (≥3).
Results.
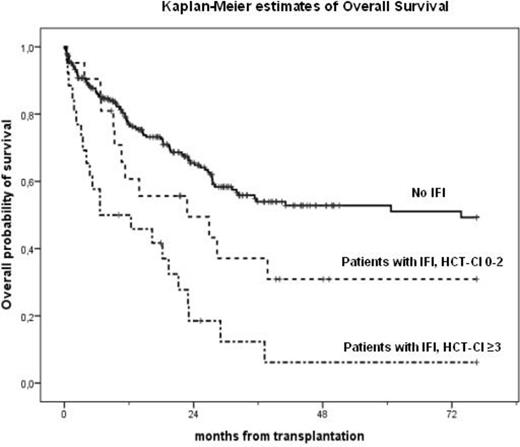
In the present cohort the median overall survival (OS) was 35 months, with a median follow-up of 24 (1-77) months from HSCT. The cumulative incidence of IFI (possible-probable-proven) at 1 year was 14% with a trend towards a higher cumulative incidence of IFI among patients with a high HCT-CI (17%) compared to patients with HCT-CI 0-2 (11%; p=0.052). A significant better 2-year OS (67%) was observed in patients who received HSCT from matched related donor (MRD) compared to alternative donors (matched unrelated donor, MUD, and haploidentical HSCT) who showed a 2-year OS of 56% (p= 0.004). A statistically significant difference in 2-year OS was also observed in patients transplanted in early disease phase compared to patients who received the graft in advanced phase, 70% vs 53%, respectively (p=0.007). Moreover, patients with low-intermediate HCT-CI showed a significant better 2-year OS (70%) compared to patients with high HCT-CI (46%; p<0.001). Similarly, survival at 2 years post-HSCT, was significantly higher in patients with no evidence of IFI (66%) compared to patients with IFI (33%; p<0.001). Dividing the patients with IFI on the basis of HCT-CI score, the subgroup with high HCT-CI had a significantly shorter 2-year OS (19%) than patients with an IFI and low or intermediate HCT-CI (50%; p<0.001) (figure 1). Univariate analysis for OS demonstrated that age ≥ 55 years (p=0.045), the use of an alternative donor (p=0.004), an advanced disease phase at HSCT (p=0.041) and the presence of IFI (p<0.001) were significantly associated with a worse outcome. Moreover, in univariate analysis the HCT-CI score showed a significant association with OS (p<0.001), with a hazard ratio of 1.89 in the group with a HCT-CI score ≥ 3. Adjusting in a multivariate model, the use of an alternative donor (HR 1.70; p=0.009), a HCT-CI ≥ 3 (HR 1.82; p=0.001) and the presence of IFI (HR 2.22; p<0.001) were significant predictors of worse 2-year OS. Further, a pre-transplant high HCT-CI (HR 2.71; p <0.001) and the development of IFI (HR 3.69; p<0.001) were significant independent risk factors for non-relapse mortality (NRM), while IFI was a significant predictor for infectious-related mortality (IRM) with a HR 3.37 (p= 0.010).
Conclusions.
The results of the present study highlight the additional prognostic value of HCT-CI on the outcome of HSCT patients who developed an IFI. These findings may lead to the consideration of whether patients with a high HCT-CI score may be candidates for an intensive diagnostic work-up or a specific antifungal prophylaxis. Larger studies are required to address this issue.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.